
Antibodies are complex proteins produced by B-cells during the adaptive immune response. They recognize and bind foreign biomolecules to confer protection, for example by triggering cytotoxic T lymphocytes to kill cells that have been infected with a bacterium or by blocking viral surface proteins to prevent host cell entry. Because antibodies often demonstrate high specificity for their target antigens, they have been widely exploited as reagents for scientific research, where they are categorized as being either polyclonal or monoclonal according to the method of production. Polyclonal and monoclonal antibodies offer distinct advantages and disadvantages that should be carefully considered during reagent selection.
How are polyclonal and monoclonal antibodies produced?
To understand how polyclonals and monoclonals differ, it’s important to remember that every B-cell expresses a unique antibody molecule on its surface. This diversity occurs early in B-cell development and takes place via mechanisms including V-D-J recombination resulting in ‘junctional sequence diversification. Upon encountering its target antigen, the surface-bound antibody prompts the B-cell to proliferate, giving rise to populations of B-cells, some of which express and secrete the same antibody molecule – a process known as clonal expansion.
Now, consider the fact that the target antigen is likely expressed on the surface of a foreign invader such as a bacterium. Other B-cells will recognize and bind distinct regions (epitopes) of the target antigen, with each resultant B-cell population producing a separate antibody. By collecting blood from an immunized host animal and purifying the antibodies from the sera, one would end up with a mixture of antibodies to the target of interest – this is referred to as being polyclonal since the antibody clones it comprises all recognize different epitopes.
An alternative approach to purifying antibodies from blood is to extract the spleen of an immunized animal and fuse the B-cells it contains with immortal myeloma cells to produce hybridoma. This method was developed by Kohler and Milstein in 1975 and led them to receive a Nobel Prize. By diluting the hybridomas and distributing across microtiter plates such that each well receives just a single cell. Individual clones can be isolated and expanded so that allowing antibodies to be purified from the tissue culture supernatant for as long as the cell line is kept alive (Figure 1). Each unique antibody population can then be evaluated for suitability, with the best-performing clones being archived to ensure future supply.
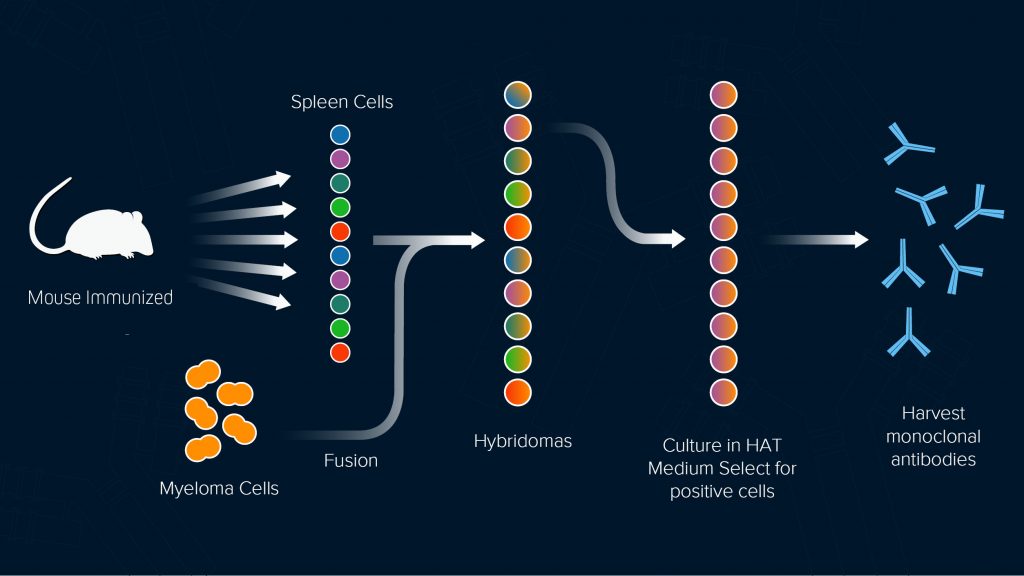
Figure 1. Hybridoma technology.
What are the advantages and disadvantages of polyclonal antibodies?
Polyclonal antibodies offer several advantages. First, because they are relatively easy to produce, they are rapidly available for studying novel targets – like SARS-CoV-2. Polyclonals may also provide signal amplification due to their heterogeneous population simultaneously being able to recognize multiple epitopes. This heightened sensitivity makes them likely to perform in a broad range of research applications; where an epitope is altered by sample processing, epitope redundancy means that the target should still be detected.
Many polyclonals are produced in rabbit hosts, where the unique nature of the rabbit immune system enables antibodies to be developed against challenging targets such as small peptide antigens. Where larger hosts such as goats or sheep are used, polyclonals can be generated in volumes suitable to support a long-term application in either research or diagnostics.
A main drawback of polyclonal antibodies is that they are available in only finite supply, correlating to the lifespan of the host animal. Antibody performance can also vary from lot-to-lot, requiring researchers to re-optimize experimental conditions each time a new batch is received. Lastly, because polyclonal antibodies are heterogeneous, their use may be associated with an increased risk of non-specific binding, although some antibody manufacturers (including Jackson ImmunoResearch) address this issue with cross-adsorption chromatography.
What are the advantages and disadvantages of monoclonal antibodies?
A major advantage of monoclonal antibodies is their specificity, which is attained by characterizing individual clones during antibody development. Monoclonals also demonstrate highly consistent performance that helps safeguard experimental reproducibility. A further benefit of monoclonal antibodies is that they are available in unlimited supply, with their production avoiding the need for successive animal immunizations and being readily scalable to meet demand.
Historically, monoclonals have been more expensive than polyclonals due to the more time-consuming development process and manufacturing costs. However, this gap is slowly closing, and the use of both polyclonal and monoclonal antibody reagents for research continues to grow. Finally, monoclonal antibodies may suffer from paratope inactivation if critical residues of the monoclonal are modified during conjugation to reporter molecules such as fluorescent dyes of enzymes.
| Polyclonal | Monoclonal | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Can you use polyclonal and monoclonal antibodies together?
Monoclonal and polyclonal antibodies are often used in combination as detection pairs. Monoclonal antibodies are specific to a single binding site on the protein of interest. This characteristic is often highly desirable when selecting a primary antibody, as detection can be more predictable over repeated assays. The drawback of this is that typically only one monoclonal primary antibody can bind an individual target molecule presenting a single epitope. In comparison, polyclonal primary antibodies can recognize multiple epitopes on the target which can be an advantage. A conjugated polyclonal secondary antibody that has specificity for multiple epitopes on the primary antibody is usually preferred for detection, as the signal amplification can increase the sensitivity of the assay. In theory, combining polyclonal primaries with polyclonal secondaries offers the highest possible signal amplification.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications. Our portfolio includes a broad range of key reagents for routine screening, specialist immunoassays and staining experiments. With a wide selection of specificities and formats conjugated to reporter enzymes, fluorophores, or other biomolecules, and a comprehensive suite of secondary antibodies to meet exacting research requirements. All our products are backed by expert technical support to help you achieve reliable results, every time.
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



