Lateral Flow Immunoassay: Methodology, Applications, and Considerations for Use
Lateral flow immunoassay (LFIA) is a membrane-based technique for detecting specific analytes in complex samples. Advantages of LFIA are that it is relatively inexpensive to develop and manufacture, easy to use, and provides rapid results. Additionally, because LFIA does not require refrigerated storage, it is well-suited to use in developing countries, remote geographies, and settings with limited facilities. For these reasons, LFIA is seeing increased uptake for a broad range of applications.

How does a lateral flow immunoassay work?
A typical LFIA comprises several core components, all of which are mounted on an inert backing material and housed in a plastic case (either a cassette or a dipstick format) for easier handling. The first of these is a sample pad, an adsorbent pad permeated with salts and surfactants to promote analyte detection, which is where the sample is applied. This is followed by a conjugate release pad containing analyte-specific antibodies that are labeled with detection moieties such as colloidal gold or colored latex beads. After the conjugate release pad comes a porous membrane (usually nitrocellulose) where further antibodies are immobilized in one or more lines. Commonly, both a test line and a control line are included on the membrane, which respectively function to capture the analyte and ensure the LFIA is performing correctly. The final component of the LFIA is an absorbent pad, which serves to keep the sample moving (via capillary action) and prevent backflow. As the sample migrates through the LFIA, target accumulation gives rise to a signal that can normally be seen with the naked eye.
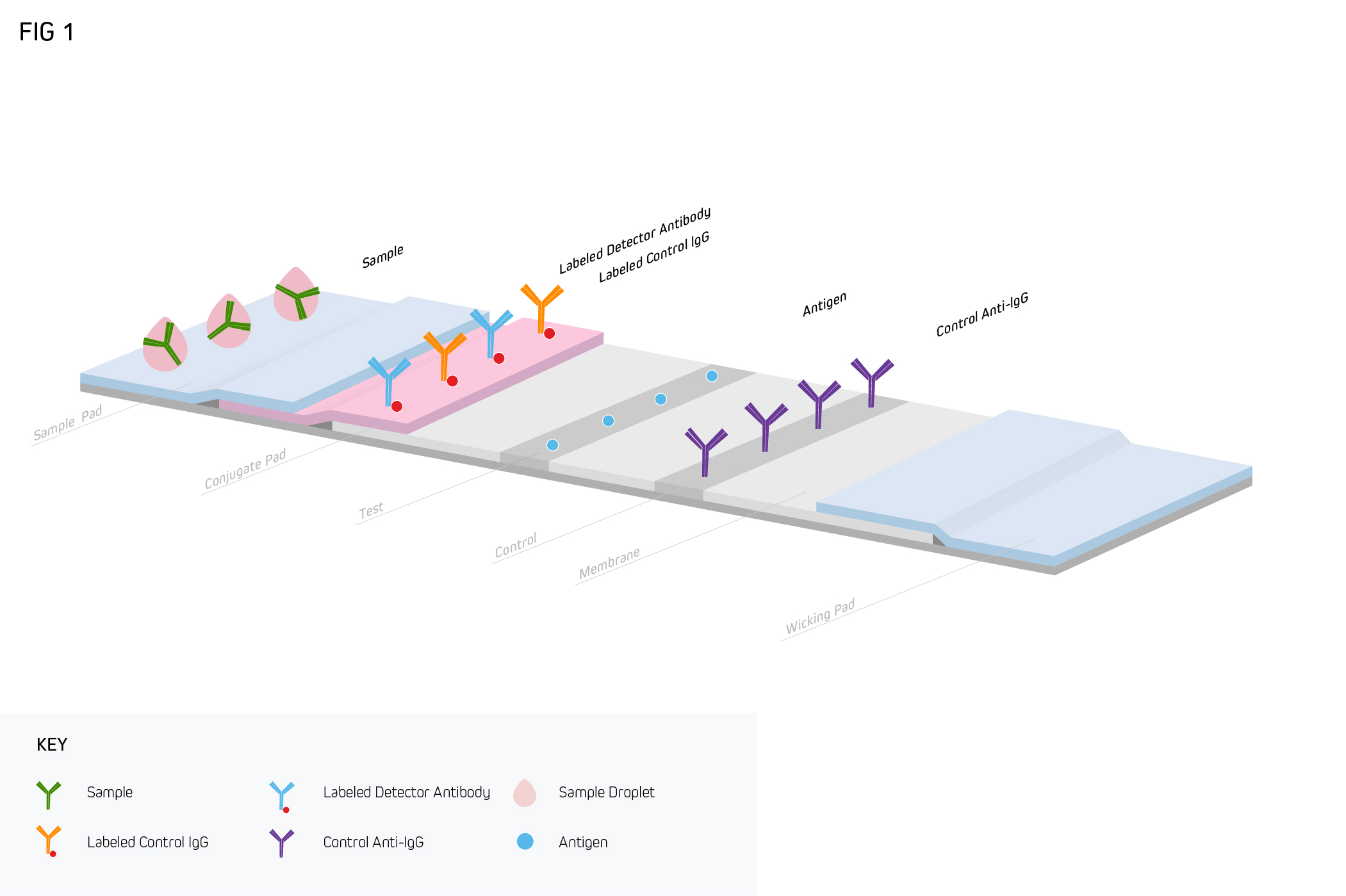
How are lateral flow immunoassays used?
Because LFIAs can be adapted for the detection of almost any analyte, their use spans multiple fields. Within a healthcare setting, LFIAs are used to confirm pregnancy (e.g., by detecting the human chorionic gonadotropin hormone in urine), diagnose infection (e.g., by detecting SARS-CoV-2, the virus responsible for COVID-19, in nasopharyngeal swab samples), and establish whether a patient has an allergy (e.g., by detecting total IgE, or allergen-specific IgE, in blood). LFIAs are also being developed for numerous disease biomarkers, including prostate-specific antigen (PSA), which is often elevated by prostate cancer and other prostate disorders, and C-reactive protein (CRP), a major inflammatory marker. Outside of patient care, other applications of LFIA include screening for animal diseases, monitoring water quality, and safeguarding food production by testing for harmful contaminants such as bacterial toxins.
What are some key considerations for setting up a lateral flow immunoassay?
Although LFIAs can be configured in several different ways, they most often employ a sandwich assay format for highly specific detection of protein analytes. In this arrangement, a labeled analyte-specific antibody is loaded onto the conjugate pad and another analyte-specific antibody is immobilized on the membrane. Critically, these antibodies should each recognize a distinct epitope to avoid competition for target binding.
A popular approach is to load the conjugate pad with a monoclonal antibody and immobilize a polyclonal antibody on the nitrocellulose membrane, where the latter can increase assay sensitivity through its binding to multiple epitopes. In this scenario, sourcing the polyclonal antibody from a larger host species such as donkey or goat means bigger batch sizes, translating to greater reproducibility over time compared to using antibodies from smaller animals like rats or rabbits.
Where the LFIA will be used for detecting antibodies rather than protein antigens (e.g., during allergy testing or to determine whether a patient has generated an immune response to an infection), the conjugate pad will usually be loaded with the labeled analyte. Here, either a monoclonal or a polyclonal can be immobilized on the membrane for target capture, provided it has been shown to exhibit high specificity for the analyte in question.
When developing antibody-based LFIA controls, it is suggested that, where possible, researchers source antibody reagents from host species that are unrelated to the test sample. For example, loading the conjugate pad with chicken IgY antibodies that are labeled with gold nanoparticles and using a goat anti-chicken IgY antibody as the control line is a tried and trusted approach to prevent unwanted cross-reactivities when working with human samples.
The choice of conjugate is a key consideration for LFIA. While gold nanoparticles remain the leading LFIA detection moiety due to their low cost, strong color, and consistent size, other modalities are increasingly being used. These include latex beads, which are available in a range of colors that can make interpreting multiplexed data easier; fluorophores such as fluorescent proteins or Europium particles, which can enhance assay sensitivity and enable quantification provided a dedicated strip reader is available for measurement; and newer strategies aimed at boosting the signal from colloidal gold or gold nanoparticle-enzyme conjugates.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications, including lateral flow immunoassay. We offer an extensive range of Anti-Human antibodies available as 40nm Gold conjugates, as well as many different antibody-fluorophore conjugates, all of which undergo rigorous validation before being released for use.
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |


