Combining the exquisite specificity our minimally cross-reactive antibodies have always offered with the unique penetrative capabilities of the small VHH fragment format, JIR AffiniPure-VHH™ Secondary Antibodies enable high precision staining. Read our article to explore how they behave in mouse brain tissue.
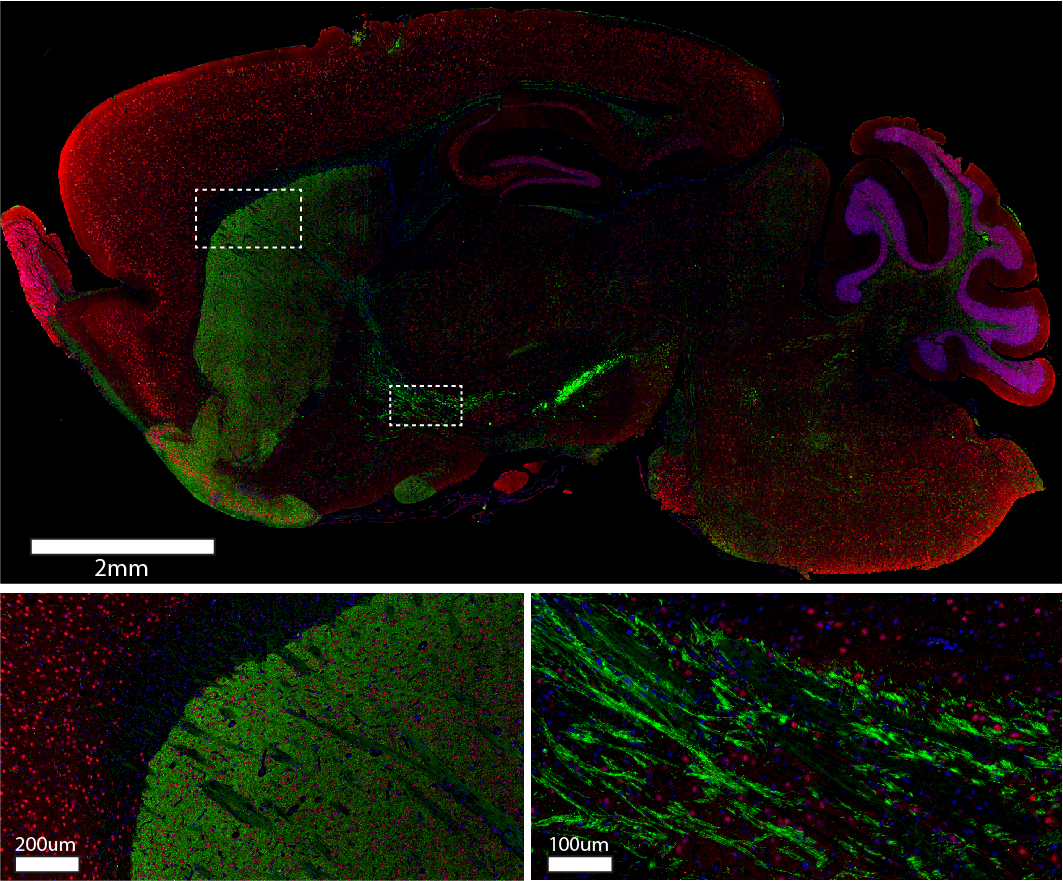
Staining brain tissue
Staining any tissue requires experimental considerations; sometimes, it’s about the potential for cross-reactivity, sometimes it’s the permeability of the reagents, and sometimes it’s about finding the right reporter molecule. When staining multiple targets, all three considerations are critical to generating results that illustrate clear, impactful, and visually appealing findings. Whether you want to determine protein expression, where it’s localized, or if it colocalizes with another molecule, JIR AffiniPure-VHH™ Secondary Antibodies can help you amplify the detail.

Sagittal sections of mouse brain were blocked with 5% normal alpaca serum in Tris buffer. Primary antibodies were applied at 4C overnight at the following dilutions: rabbit anti-Tyrosine Hydroxylase (Abcam #ab112) at a 1:250 dilution; mouse anti-NeuN (Millipore #MAB377) at a 1:100 dilution. The following secondary antibodies were applied at room temperature for one hour at 1:220 dilutions: Alexa Fluor® 488 VHH Fragment Alpaca Anti-Rabbit IgG (H+L) (611-544-215); Cy3 VHH Fragment Alpaca Anti-Mouse IgG (H+L) (615-164-214). Nuclei of cells were visualized using DAPI (blue). Images were obtained from slides created and provided by UNC’s Histology Research Core Facility.
Polyclonal antibodies let you amplify the detail
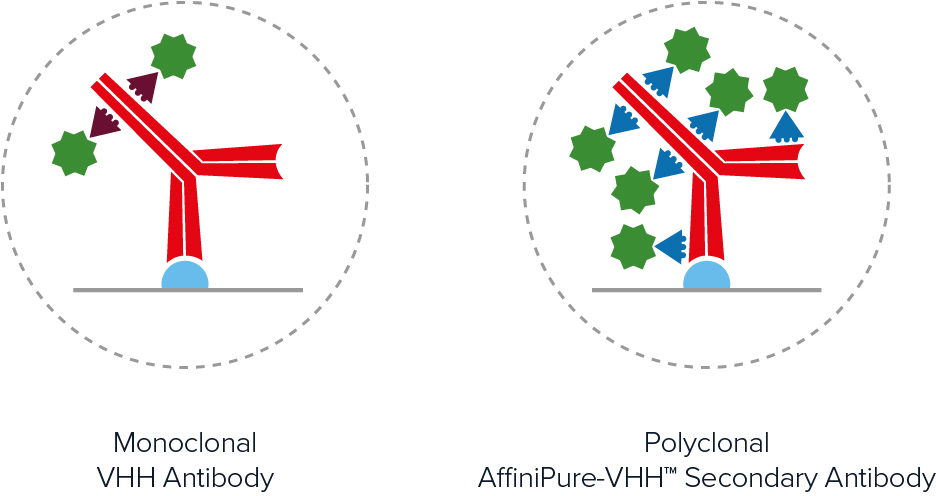
Polyclonal AffiniPure-VHH™ Secondary Antibodies offer excellent sensitivity through signal amplification. As seen in Figure 2, polyclonal antibodies achieve higher labeling efficiency than monoclonal antibodies by binding to multiple locations across the primary antibody, generating a brighter signal.
Min-X for exquisite differentiation
When visualizing multiple targets, cross-adsorbed antibodies allow you to build up a multicolored image without worrying about the off-target signal from cross-reacting antibodies. AffiniPure-VHH™ Secondary Antibodies are available cross-adsorbed (min X) to commonly used species which can reduce background and enhance specificity, enabling you to experience exquisite differentiation of target proteins.
Small means better penetration
One-tenth the size of conventional antibodies, these tiny antibody fragments offer excellent tissue penetration allowing you to reduce incubations or access targets that require specialized permeabilization treatment. Nanobodies lack an Fc effector domain which allows enhanced clearance and aids live-cell imaging techniques such as Immuno-PET.
Better choice – choose from the whole spectrum
Don’t be limited by conjugate availability; when a directly conjugated antibody isn’t available, JIR AffiniPure-VHH™ Secondary Antibodies give you access to a range of dyes without compromising resolution. We offer DyLight, Alexa Fluor®, and Cyanine™ dyes covering the spectrum, so you can choose suitable conjugates for your filter set.
References:
- Carrington, G., Tomlinson, D. and Peckham, M., 2019. Exploiting nanobodies and Affimers for superresolution imaging in light microscopy. Molecular Biology of the Cell, 30(22), pp.2737-2740.
- Chakravarty, R., Goel, S., and Cai, W., 2014. Nanobody: The “Magic Bullet” for Molecular Imaging?. Theranostics, 4(4), pp.386-398.
- De Groeve, K., Deschacht, N., De Koninck, C., Caveliers, V., Lahoutte, T., Devoogdt, N., Muyldermans, S., De Baetselier, P. and Raes, G., 2010. Nanobodies as Tools for In Vivo Imaging of Specific Immune Cell Types. Journal of Nuclear Medicine, 51(5), pp.782-789.
- Debie, P., Lafont, C., Defrise, M., Hansen, I., van Willigen, D., van Leeuwen, F., Gijsbers, R., D’Huyvetter, M., Devoogdt, N., Lahoutte, T., Mollard, P. and Hernot, S., 2020. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. Journal of Controlled Release, 317, pp.34-42.
- Erreni, M., Schorn, T., D’Autilia, F. and Doni, A., 2020. Nanobodies as Versatile Tool for Multiscale Imaging Modalities. Biomolecules, 10(12), p.1695.
- Hassanzadeh-Ghassabeh, G., Devoogdt, N., De Pauw, P., Vincke, C. and Muyldermans, S., 2013. Nanobodies and their potential applications. Nanomedicine, 8(6), pp.1013-1026.
- Jovčevska, I. and Muyldermans, S., 2019. The Therapeutic Potential of Nanobodies. BioDrugs, 34(1), pp.11-26.
- Krasniqi, A., D’Huyvetter, M., Devoogdt, N., Frejd, F., Sörensen, J., Orlova, A., Keyaerts, M. and Tolmachev, V., 2018. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. Journal of Nuclear Medicine, 59(6), pp.885-891.
- Stark, H. Urlaub, and D. Görlich. 2015. Nanobodies: Site-specific labeling for super-resolution imaging, rapid epitope-mapping, and native protein complex isolation. eLife. 4:e11349. https://doi.org/10.7554/eLife.11349
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



