
Fluorophores are essential to many different applications, from flow-cytometry, confocal and super-resolution microscopy to Western blotting and ELISA. From recombinant fluorescent proteins to the hugely popular fluorescent organic dyes when conjugated with secondary antibodies, they enable the visualization of the target antigen. Here we explore the history and progress of the application of fluorescent molecules in science.

Fluorescent proteins
Although the discovery of bioluminescence dates back to the 1500s, it wasn’t until 1852 that the term “fluorescence” came into use. “Fluorescence” was coined by George Gabriel Stokes (Web ref:1, Kalyuzhny 2016) after discovering that the mineral fluorite emitted visible light after exposure to ultraviolet light. A century later, in 1955, Green Fluorescent protein (GFP) was identified in the Jellyfish Aequorea victoria by Davenport & Nichol (2), and later isolated by Osama Shimomura in 1962. However, it wasn’t until 1996, when Roger Tsien’s group identified a point mutation in GFP (S65T), making the protein practical for research applications, that GFP’s research possibilities were realized (3).
Since then, with optimization, the recombinant protein GFP has been modified to yield many variations in color, from cyan and the original green through to the reds of mCherry and mTomato, with improvements in photostability, brightness, and experimental vigor.
GFP provided a starting block for the study of biological processes in living cells and the development of techniques that wouldn’t be possible without the unique properties of color. The impact of GFP on the world of research was highlighted by the award of the Nobel Prize for Chemistry in 2008 to Shimomura, Tsien, and Chalfie for their work on GFP (4).
GFP and its analogs can be “coded” into the protein sequence and “expressed” as part of the protein at points deemed appropriate based on structure and known or predicted function. Expression of GFP fusion proteins can present challenges that can limit their use. Although they may enable experiments such as co-localization or even Förster Resonance Energy Transfer (FRET), allowing stoichiometrical characterization, such experiments require careful development.
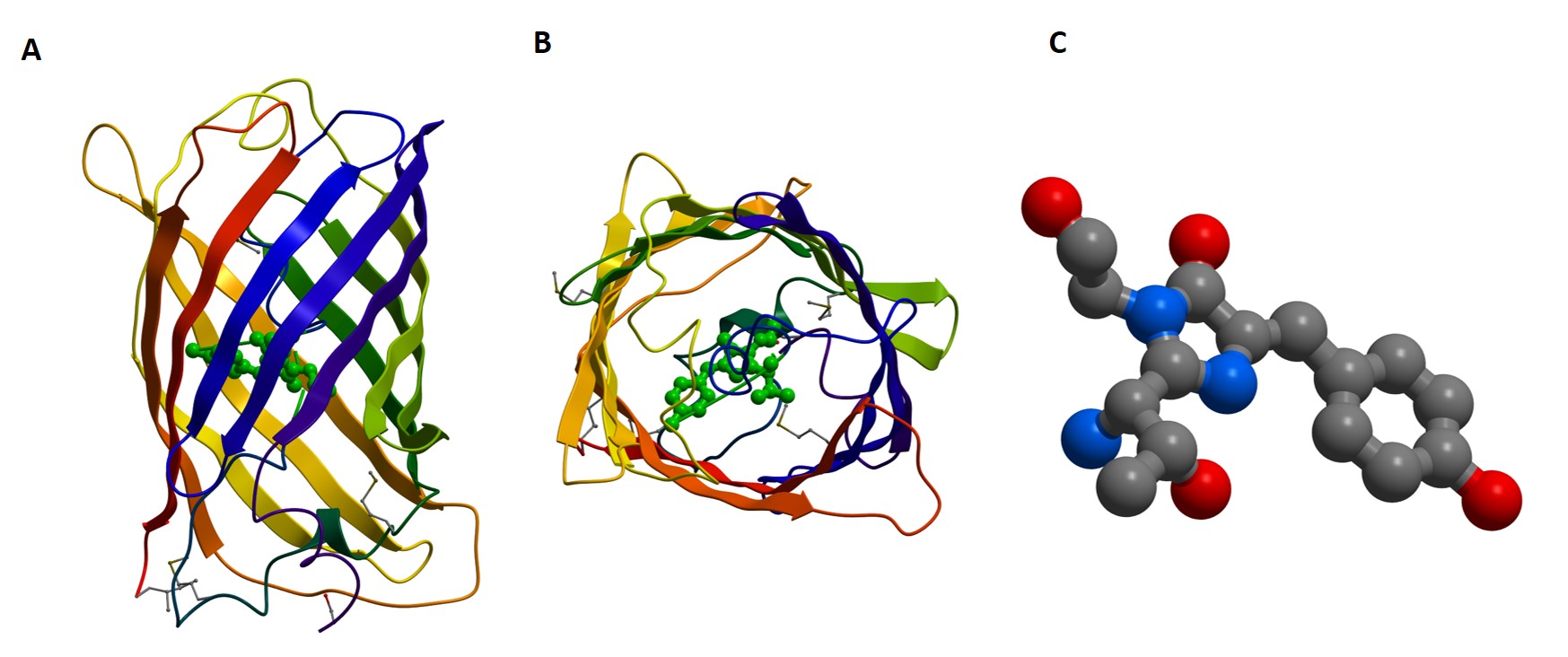
Transfection of plasmids coding for the fluorescent protein and the gene of interest can be time-consuming, requiring optimization, and the fluorophore may change the behavior of the protein of interest, negating the value of the experiment.
Fluorescence, however, was a captivating characteristic that, if harnessed, the potential value of which scientists couldn’t ignore. This led researchers to develop other methods to add fluorophores to their molecules of interest without the artifacts incurred by expressing a recombinant fluorescent protein.
Organic dye and fluorescent protein conjugates
The antigen:antibody interaction is a tractable way of labeling proteins in situ on live cells, as well as adding multiple fluorescent molecules per antigen–amplifying signal. Fluorescent molecules suitable for labeling have been developed from the phycobiliproteins of photosynthetic algae (8) to the much smaller organic dyes like Alexa Fluor® and Cyanine dyes, and much smaller fluorescent particles like Quantum dots. These other molecules have increased the range of colors available to scientists and have enhanced the qualities of the fluorophores available, allowing their application to be diversified into numerous techniques.
Organic dyes and fluorescent proteins can be used as fluorescent probes conjugated (or “tagged”) to antibodies, allowing the researcher to take advantage of many of the benefits of in situ staining over recombinant expression of fluorophores.
The first conjugated fluorophores to appear were FITC (fluorescein isothiocyanate) and TRITC (tetramethyl rhodamine isothiocyanate) (7) back in the 1950s. As computing power has increased to cope with the processing demands of multi-channel experiments and our optical techniques have developed, the range of available dyes has exploded.
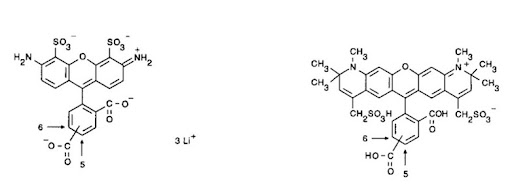
Conjugated fluorescent probes offer numerous advantages to recombinant proteins. They can have less potential for steric interference and expression artifacts, as well as offer the signal enhancement benefits of indirect detection – by binding more dye molecules per antigen molecule – potentially increasing the brightness – adding sensitivity to the assay. The antibody conjugates can also offer improved photostability, decreased photobleaching, and a more “user-friendly” format. Numerous fluorescent conjugates are available, spanning the spectrum from blue through to infrared.
Jackson ImmunoResearch offers a comprehensive collection of conjugates spanning the entire spectrum. Phycobiliproteins – Phycoerythrin (PE), Peridinin chlorophyll protein (PerCP), and Allophycocyanin (APC), Alexa Fluor®, Brilliant Violet, and Cyanine dyes conjugated to Jackson Immunoresearch’s secondary antibodies offer a kaleidoscope of possibility.
References:
- https://www.britannica.com/biography/Sir-George-Gabriel-Stokes-1st-Baronet
- Davenport D, Nicol JAC. (1955). Luminescence in Hydromedusae. Proc. R. Soc. Lond. B 144, 399–411.
-
SHIMOMURA, O., JOHNSON, F. H., & SAIGA, Y. (1962). Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. Journal of cellular and comparative physiology, 59, 223–239. https://doi.org/10.1002/jcp.1030590302
- Heim R, Tsien RY. (1996). Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Current Biology. 1996 Feb 1;6(2):178-82.
- Roger Y. Tsien (2010) The 2009 Lindau Nobel Laureate Meeting: Roger Y. Tsien, Chemistry 2008. J Vis Exp. 2010; (35): 1575.
- Ormo, M., Cubitt, A.B., Kallio, K., Gross, L.A., Tsien, R.Y., Remington, S.J.(1996) Crystal structure of the Aequorea victoria green fluorescent protein. Science 273: 1392-1395
- Nataliya Panchuk-Voloshina et al. Alexa Dyes, a Series of New Fluorescent Dyes that Yield Exceptionally Bright, Photostable Conjugates. J Histochem Cytochem 1999;47:1179-1188
- RIGGS JL, SEIWALD RJ, BURCKHALTER JH, DOWNS CM, METCALF TG. (1958). Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958 Nov-Dec;34(6):1081–1097.
- COONS AH, KAPLAN MH. (1950) Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1-13.
- Morisset. K & Walter.B (1984), Phycobiliproteins — characterization of coloured algal proteins by a simple electrophoretic procedure. Biochemical Education (12.4).
- Kalyuzhany (2016) Immunohistochemistry – Essential elements and beyond. Springer. Switzerland
| Learn more: | Do more: |
|---|---|
| Indirect and direct Western blotting | Exhibition Schedule |
| Chemiluminescence western blotting | Cite and Win |
| An Introduction to Expansion Microscopy | Past Competition Winners |



