We caught up with Derek Sung, Immunofluorescence guru, Ph.D. student, and the brains behind the outstanding Instagram identity – @immunofluorescence. Busy generating results for his Ph.D., he’s found time to test-drive our New AffiniPure-VHH™ Secondary Antibodies. Read what he has been up to and find out how the AffiniPure-VHH™ Secondary Antibodies behaved in his hands.
What have you been working on?
“I’m currently working on a few projects related to the placenta and how defects in the placenta influence organ development in the embryo, particularly the heart, brain, liver, and blood vessels. It’s a lot of fun because I can image all these different organs and see them at different stages of development.”
What did you like about the (AffiniPure-VHH™ Secondary) antibodies?
“I liked how there was no learning curve as someone who uses mainly goat and donkey secondary antibodies. The steps are almost exactly the same, and the signal is extremely high and long-lasting (the signal is still very high even three weeks later).”
Were there any improvements you could see ?
“I did not notice any appreciable differences in resolution, but this is likely due to my imaging modality (widefield fluorescence). I think the size of the antibody will be most advantageous with super-resolution methods, such as SIM, TIRF, and STORM.”

Derek Sung, MD/PhD Student · University of Pennsylvania School of Medicine
“I’ve tested out the products and am happy to say that they worked GREAT in my hands.”
Are there any applications you can see them being useful for, or experiments you’d like to do with them?
“I think the nanobodies will be particularly useful in whole-mount immunofluorescence of cleared tissue. One of the major barriers to high-quality whole-mount immunofluorescence is the robust penetration of antibodies due to their size. Given that the nanobodies are ten times smaller than donkey or goat immunoglobulins, I think this will be really useful in staining entire tissues and organs.”
Was blocking important?
“I only tested the products after blocking with 10% alpaca serum, but I had minimal background signal, so I would recommend blocking for optimal results.”
Are there any other specificities or fluors you’d like us to add?
“Other common species for primary antibodies, such as goat and rat, would be great!”
What are your plans for the future?
“I’m currently wrapping up my projects and preparing to defend my thesis in August. After that, I’ll return to medical school to finish my MD. I hope to one-day practice medicine while running a lab and continuing to study reproductive vascular biology.”
“I’ve been getting a lot of great messages regarding the antibodies! I think it’ll be a great product and resource for the community.”
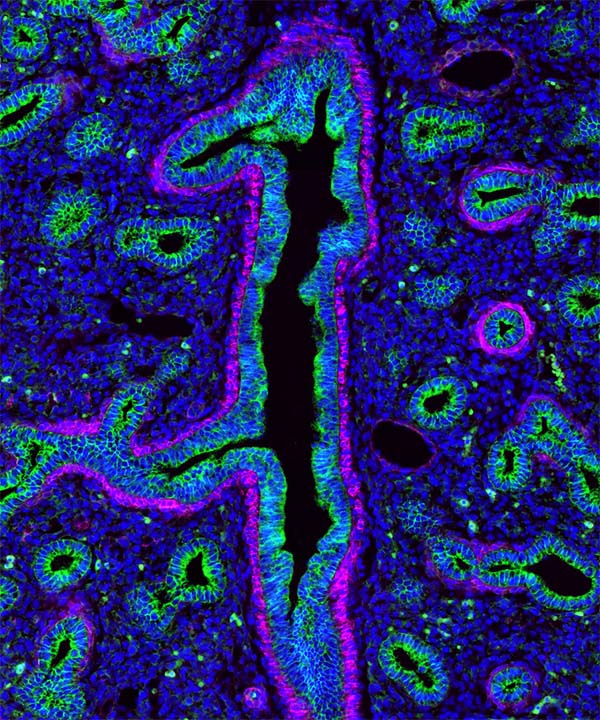
The following images were made by Derek using JIR AffiniPure-VHH™ Secondary Antibodies. “The first three images are of the lung, small intestine, and tongue/palate all from a developing embryo. I stained these with E-cadherin (green) and alpha-smooth muscle actin (magenta). In each image, the E-cadherin outlines the epithelial cells really nicely and is surrounded by a ring of smooth muscle. You can see the developing airways in the lungs, the lining of the small intestine, and the layer of cells covering the tongue and palate.”
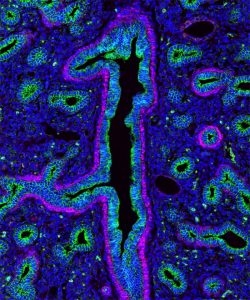
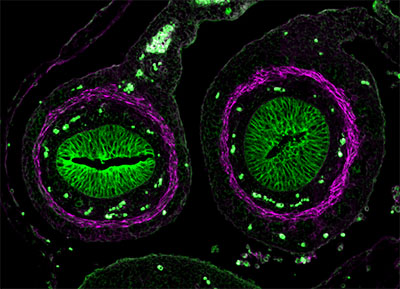
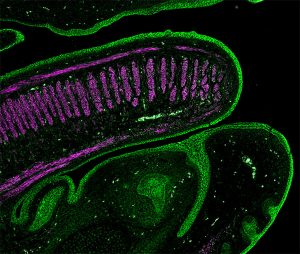
These images used 611-474-215 (DyLight 405 VHH Fragment Alpaca Anti-Rabbit) and 615-544-214 (Alexa Fluor® 488 VHH Fragment Alpaca Anti-Mouse) and blocked with 10% Alpaca serum (028-000-001).
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |


