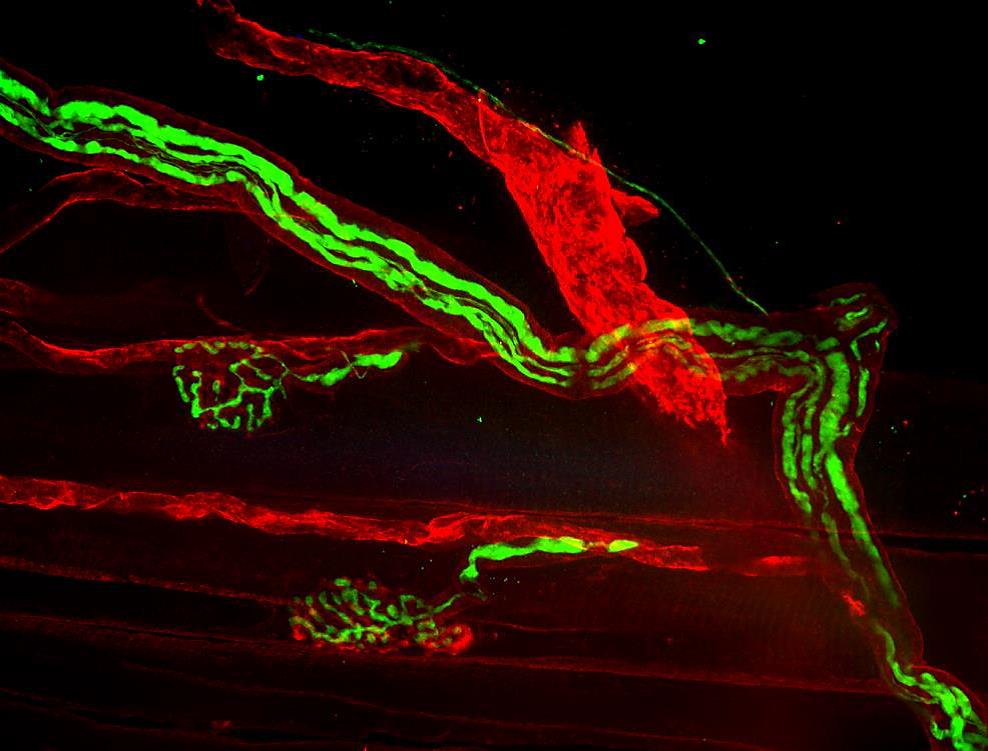
Antibodies labeled with fluorescent dyes are essential tools for a broad range of research techniques, especially those requiring multiplexed detection. In recent years, dyes that emit in the far-red and near-infrared (NIR) regions have seen increased use due to the advantages that they offer.

A brief history of antibody labeling
The labeling of an antibody with a dye was first reported in 1934, when John Marrack coupled antibodies to tetrazotized benzidine1. However, because only a faint color was produced when the antibodies precipitated in solution, his method was subsequently adapted by Coons et al. to produce a fluorescent signal.
By coupling anthracene isocyanate to anti-pneumococcal antibodies and using these to agglutinate pneumococcal bacteria in solution, Coons et al. were able to observe visible fluorescence when the sample was excited with ultraviolet light2. However, because autofluorescence prohibited similar results in infected tissue sections, they later switched to labeling with fluorescein isocyanate.
Today, a vast array of fluorescent labels is available to researchers, with products spanning the violet region of the spectrum (~400 nm) through to emissions. In addition, various tandem dyes have been developed to increase flexibility for experimental design.
Principles of fluorescence
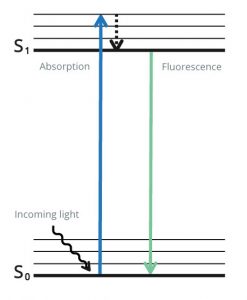
Fluorescence is produced when specialized molecules called fluorophores absorb photons of light within a specific range of wavelengths and emit them at another, longer range of wavelengths. During this process, the fluorophore’s electrons are excited from a resting state to a higher energy state, and it is only by releasing energy that they can return to a stable condition (Figure 1). Because the energy is released as both heat and light, the resultant fluorescence has a higher wavelength than that which was used for excitation. When fluorophores are attached to antibodies, the emitted light can be used for analyte detection with techniques including flow cytometry, fluorescence microscopy, and western blot.
Key fluorophore properties
Fluorophores are characterized by several key properties. The excitation maximum and emission maximum are, respectively, the wavelengths at which light is absorbed and emitted most efficiently, while the Stokes shift is the difference between these two values. The molar extinction coefficient, which is the amount of light absorbed at a given wavelength, and the quantum yield, which is the number of photons emitted for every photon absorbed, govern fluorophore brightness.
Often, fluorophore properties are influenced by the assay environment. For example, Propidium Iodide (PI) is only weakly fluorescent in water, but produces bright fluorescence upon intercalating with DNA due to its hydrophobic surroundings. And Acridine Orange (AO) emits green fluorescence when bound to dsDNA and red fluorescence when bound to ssDNA or RNA, owing to the differing nature of the interactions.
Reasons to consider far-red and NIR dyes
One of the main reasons for using far-red and NIR dyes is to avoid non-specific background signal due to autofluorescence when performing microscopy experiments. Sources of autofluorescence include common endogenous sample components such as collagen, riboflavin, and the reduced form of nicotinamide adenine dinucleotide (NADH), as well as aldehyde fixatives such as formalin and glutaraldehyde, all of which typically emit in the blue to green spectrum.
Far-red and NIR dyes also offer a way to minimize the risk of photobleaching during live-cell imaging studies. Because far-red and NIR dyes are excited with lower energy lasers than other dyes, they are less likely to photobleach. In addition, far-red and NIR dyes are popular for imaging 3-dimensional (3D) cell cultures such as spheroids and organoids, and for imaging living organisms, due to their deeper sample penetration depth.
Tips for setting up a dye panel
Fluorescently labeled antibodies are widely used for multiplexed experiments, which today cover everything from simple 2 to 3 color western blots through to sophisticated 20+ parameter flow cytometry assays. Although the complexity of panel design will vary with the type of experiment being performed, the following are some general tips for fluorophore selection:
- Choose dyes that are well resolved from one another to avoid spectral overlap (the spillover of fluorescent signal into other detectors than the primary detector)
- Assign bright fluorophores to analytes with low expression and dim fluorophores to more abundant targets
- Consider using far-red and NIR dyes to avoid non-specific background signal from autofluorescence or for performing live-cell imaging studies
- Think about introducing tandem dyes into your staining panel if the number of available lasers is likely to become restrictive
Spectra Viewer
Use the spectra viewer below to compare fluorophores.
Show Excitation and Emission Spectra
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications and offers an extensive selection of fluorescent conjugates, including far-red and NIR dyes.
References
- R. Marrack. (Medical Research Council, Special Report Series No. 230.) Pp. 194. (London: H.M. Stationery Office, 1938.) 3s. net. The Chemistry of Antigens and Antibodies. Nature142, 316 (1938). https://doi.org/10.1038/142316c0 https://www.nature.com/articles/142316c0
- Albert H. Coons, Hugh J. Creech, Norman Jones, Ernst Berliner; The Demonstration of Pneumococcal Antigen in Tissues by the Use of Fluorescent Antibody1. J Immunol1 November 1942; 45 (3): 159–170. https://doi.org/10.4049/jimmunol.45.3.159 https://journals.aai.org/jimmunol/article-abstract/45/3/159/63211/

| Learn more: | Do more: |
|---|---|
| Indirect and direct Western blotting | Exhibition Schedule |
| Chemiluminescence western blotting | Western blotting guide |
| An Introduction to Expansion Microscopy | ELISA guide |