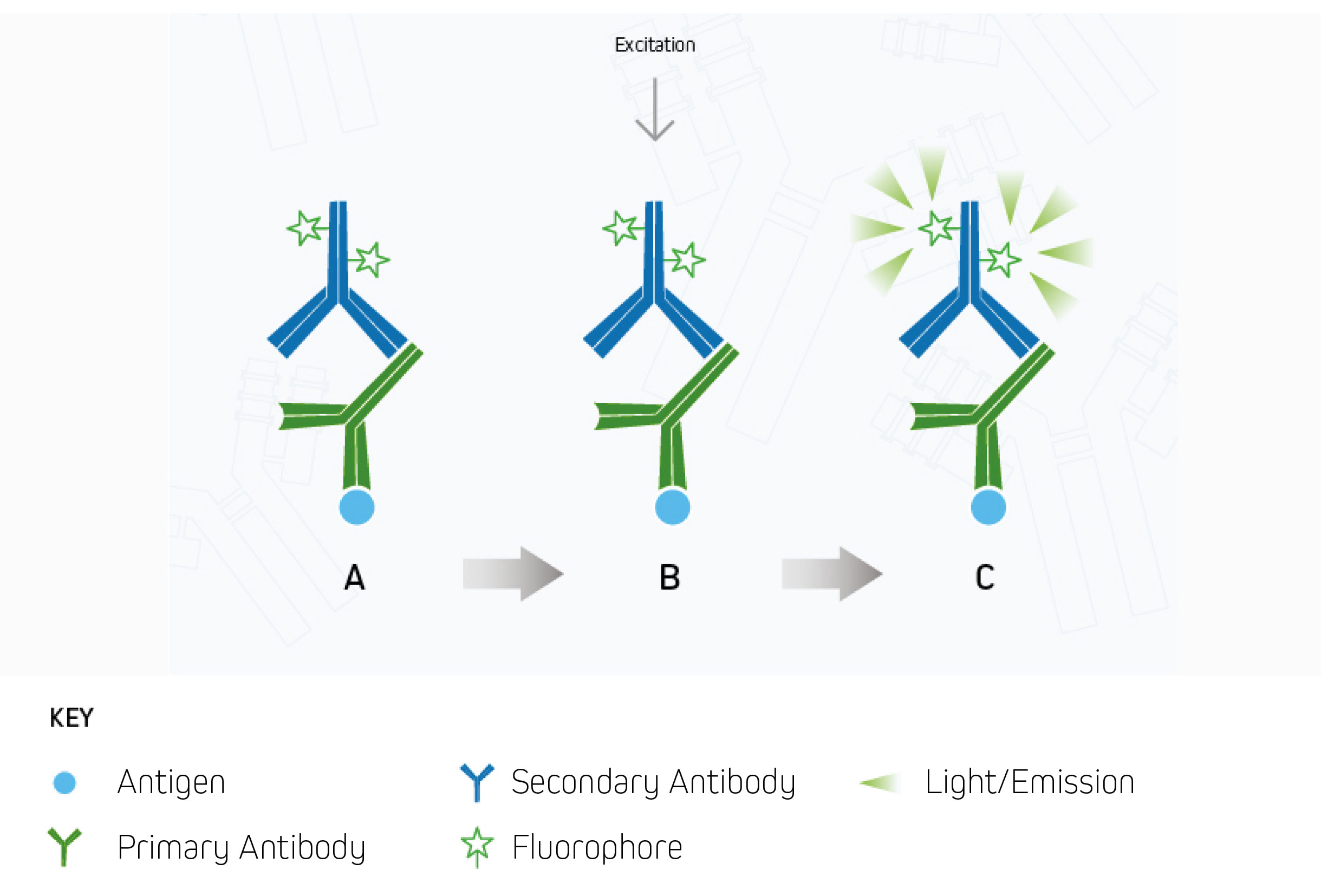
Secondary detection, also known as indirect detection, has two important advantages over direct detection. These are its capacity for signal amplification, which can increase researchers’ chances of identifying scarce analytes, and the fact that it offers access to a wider range of reporter molecules, which can provide greater flexibility for experimental design. However, a limitation of secondary detection is its higher associated risk of non-specific antibody binding, which can manifest as unwanted background signal or false positive results. This issue can be avoided by implementing the measures outlined below.
Principles of secondary detection
Antibody-based detection is classed as either direct or indirect according to the types of labeled antibodies that are used. Direct detection relies on labeled primary antibodies for identifying targets of interest, and shortens experimental workflows by requiring just a single antibody incubation step. Indirect detection instead combines unlabeled primary antibodies with labeled secondary antibodies, and can provide signal amplification as a result of multiple secondaries binding to each primary antibody. The advantages and disadvantages of each method are shown in Figure 1.
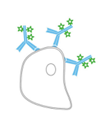
Direct Detection
Advantages
- Fewer protocol steps
- Reduced risk of non-specific signal
Disadvantages
- Labeling may compromise antibody binding
- Labeled primary antibodies can be expensive
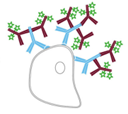
Indirect Detection
Advantages
- Can provide signal amplification as a result of multiple secondaries binding to each primary antibody
- Offers access to a wider range of reporter molecules
Disadvantages
- Experimental workflows are extended by the secondary antibody incubation and associated wash steps
- Increased risk of non-specific signal
Types of antibody labels
Secondary antibody labels include enzymes such as horseradish peroxidase (HRP) and alkaline phosphatase (AP); fluorescent proteins like R-phycoerythrin (RPE), allophycocyanin (APC), and the peridinin-chlorophyll-protein complex (PerCP); and synthetic dyes such as fluorescein isothiocyanate (FITC), the Brilliant Violet™ dyes, and the Alexa Fluor® products. In addition, secondary antibodies are labeled with biotin, gold nanoparticles, and agarose or magnetic beads, giving them utility for a broad range of research applications. Techniques that use secondary antibodies include enzyme-linked immunosorbent assay (ELISA), which enables researchers to detect an analyte in solution; western blotting, which allows for detecting a protein of interest following polyacrylamide gel electrophoresis (PAGE) and subsequent transfer to a membrane; and microscopy-based methods such as immunocytochemistry (ICC) and immunohistochemistry (IHC), which provide information about protein localization and relative abundance in cells and tissues, respectively. Secondary antibodies are also used for flow cytometry, immunoprecipitation (IP), Luminex® assays, and lateral flow immunoassays (LFIAs), as well as many other applications.
Addressing challenges for secondary detection
Non-specific binding of secondary antibodies to sample components, unintended primary antibodies, or other secondaries being used in a multiplexed experiment can generate unwanted background signal or be misinterpreted as a positive result. Endogenous immunoglobulins, enzymes, biotin, and autofluorescence can cause similar problems depending on the type of antibody label that is being used. Here are our five top tips for addressing secondary detection challenges.
- Be cautious with secondary antibody selection
When the aim of an antibody-based experiment is to detect just a single analyte, selecting the right secondary antibody is straightforward. For example, if the primary antibody was raised in a rabbit, an anti-rabbit secondary antibody should be chosen. But if several primary antibodies will be combined in a multiplexed experiment, things become more complicated. In this situation, it is recommended to select secondary antibodies that all share the same host species, if possible, to prevent them from binding to one another. If this is not feasible, using well-validated class- or subclass-specific secondary antibodies can help to increase panel size. It is also advised to choose secondary antibodies that have been cross-adsorbed against the host species of the sample, as well as against the host species of any other primary antibodies being used in the same experiment, to avoid unwanted cross-reactivities.
- Choose the right blocking agent
Bovine serum albumin (BSA) is widely used as a blocking agent. However, commercial preparations of BSA often contain contaminating bovine IgG that may serve as an antigen for cross-reacting secondary antibodies. One way of addressing this issue is to use IgG-free, protease-free BSA for blocking. When performing indirect detection, it is recommended to block with normal serum from the same host species as the secondary antibody. This is because normal serum contains endogenous antibodies that will recognize and bind any reactive sites, thus preventing unwanted secondary antibody binding further downstream.
- Consider the need for additional blocking steps
Additional blocking steps are often necessary when performing techniques such as ICC and IHC, where sources of unwanted background signal include endogenous immunoglobulins, enzymes, biotin, and autofluorescence, depending on the chosen readout. Such steps typically take place after routine blocking, prior to the addition of primary antibody reagents. Blocking of endogenous immunoglobulins is advised when using a primary antibody that shares the same host species as the sample and can be achieved using an unconjugated Fab fragment antibody. Blocking of endogenous HRP should be performed when using HRP-labeled antibodies for detection and can be accomplished by incubating samples in a dilute hydrogen peroxide solution. Similarly, blocking of endogenous Alkaline Phosphatase (AP) is required when using AP-labeled antibodies, and involves either incubating samples with levamisole or with a 1% solution of acetic acid when working with intestinal tissues. Blocking endogenous biotin is recommended when using a biotin-based detection system and is best done by sequentially incubating the sample with avidin then biotin (to block any open sites on the avidin molecule).
- Confirm secondary antibody specificity
An easy way to confirm secondary antibody specificity is to attempt labeling the ‘wrong’ primary antibodies with the secondary antibody. The use of ‘secondary antibody only’ controls (i.e., running the assay as per usual but omitting primary antibody reagents) is also recommended to ensure that non-specific secondary antibody binding is not occurring. When using fluorophore-labeled secondary antibodies, sample autofluorescence should be determined by excluding the secondary antibody incubation step. Autofluorescence is commonly seen in the UV and green channels; if autofluorescence is observed, researchers are advised to consider switching to the red and far red channels for detection. These types of tests are especially critical if the secondary antibody will be included in a multiplexed panel.
- Think about replacing labeled primary antibodies with FabuLight™ antibodies
While omitting the secondary antibody incubation step from experimental workflows can save researchers’ time, labeled primary antibodies may not always be available. FabuLight™ antibodies, consisting of labeled Fab fragments specific to the Fc region of IgG or IgM primary antibodies, were developed to provide researchers with an alternative option. Intended for quick and easy labeling of primary antibodies, FabuLights are available conjugated with 9 different fluorophores and biotin. However, because FabuLights are not cross-adsorbed against other species, researchers should note that it may still be necessary to block endogenous immunoglobulins.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications and offers an extensive range of products to advance your research. These include our cross-adsorbed secondary antibodies, subclass-specific secondary antibodies, and FabuLight™ antibodies, as well as our high-quality blocking agents.

| Learn more: | Do more: |
|---|---|
| Indirect and direct Western blotting | Exhibition Schedule |
| Chemiluminescence western blotting | Western blotting guide |
| An Introduction to Expansion Microscopy | ELISA guide |


