Super-Resolution Microscopy: Principles, Technologies, and Considerations for Reagent Selection
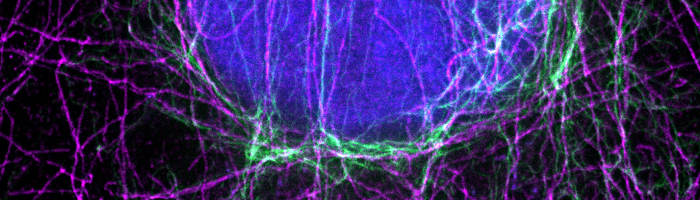
While conventional microscopy techniques are still widely used for scientific research, super-resolution microscopy (SRM) is increasingly popular for the greater level of detail it provides. Specifically, by overcoming the optical resolution limit of established microscopy methods, super-resolution microscopy allows researchers to observe sub-cellular processes such as mitochondrial function, synaptic vesicle release, and the formation of microtubule protofilaments, and can reveal how these essential functions are impacted by aging or disease. The field of super-resolution microscopy is evolving rapidly with advances in instrumentation and the development of novel reagents such as monovalent antibody fragments and brighter, more photostable fluorescent dyes. Here, we explain the underlying principles of super-resolution microscopy and highlight some of the many different methods that have been reported within the last 30 years.
What is super-resolution microscopy?
Super-resolution microscopy encompasses a growing number of techniques that overcome the optical resolution limit of conventional microscopy methods. This limit is imposed by the wavelength (λ) of light used to illuminate the sample and the numerical aperture (NA) of the microscope objective, and is defined by Abbe’s law, which states that the lateral resolution is equal to λ/(2NA) and the axial resolution is equal to 2λ/NA2. In practice, this means that, with conventional microscopy, researchers can only attain a resolution of around 250 nm in the x and y directions and 470-670 nm in the z direction1. Super-resolution microscopy is capable of much lower resolutions, in some cases down to just a few nanometers, although the exact value will vary depending on the method being used.
How are SRM techniques categorized?
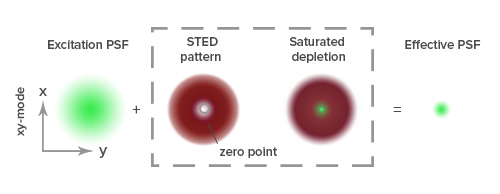
Super-resolution microscopy techniques can be broadly divided into two main categories. The first of these, known as ensemble techniques, reduce the diameter of the point spread function (PSF), which is the fixed size of the spread of a single point of light following its diffraction through a microscope, commonly defined by the Rayleigh criterion (R=0.61λ/NA). To do this, ensemble techniques restrict the production of fluorescent signals to a single focal point within the PSF by forcing any peripheral fluorophores to immediately return to a ground state following excitation1,2. Contemporary ensemble techniques include Stimulated Emission Depletion Microscopy (STED), Ground-State Depletion Microscopy (GSD), and Structured Illumination Microscopy (SIM), which were respectively reported to resolve 35 nm, 15 nm, and ~100 nm when they were first described in the literature3,4,5.
Single-molecule localization microscopy (SMLM) techniques instead work by detecting small numbers of individual fluorophores at different points in time before combining all of the data to construct an image. They rely on the use of photoswitchable fluorophores that can be interchanged between a long-lived dark off-state and a bright on-state, and allow for 3-dimensional (3D) imaging by analyzing both the position of each fluorophore and the shape of its PSF6. Examples of SMLM techniques include Photo-Activated Localization Microscopy (PALM) and Stochastic Optical Reconstruction Microscopy (STORM), as well as direct STORM (dSTORM), which improves on the original STORM method by replacing matched pairs of activator and reporter dyes with a single fluorophore7,8,9. Resolutions in the region of 20 nm have been reported for SMLM, although this value looks set to become lower.
Fluorophore considerations for super-resolution microscopy
When performing ensemble techniques such as STED, GSD, and SIM, it is recommended that researchers select fluorophores which are resistant to photobleaching, have high quantum yields, and are small enough to provide a sufficient labeling density for detecting the target of interest. Dyes that are useful for these methods include Alexa Fluor® 488, FITC, and Alexa Fluor® 594, as well as the abberior STAR dyes, which are especially valuable for STED. For SMLM techniques like PALM, STORM, and dSTORM, fluorophores should again be very bright (have high quantum yields) and should generate enough photons to reliably produce tight Gaussian distributions (narrow emission spectra) and ensure high precision. Examples of dyes that can be used for SMLM include many of the Alexa Fluor® dyes (e.g., Alexa Fluor® 488, 647, and 680), as well as Cy™ dyes such as Cy™5.
How can antibody fragments benefit super-resolution microscopy?
Most super-resolution microscopy techniques were developed using fluorophore-labeled antibodies. However, the large size of whole antibody molecules (10–15 nm) results in fluorophores being distant from the target, while antibody multivalency risks clustering, which could complicate downstream analysis10. Smaller, monovalent alternatives such as antibody fragments (Fabs) and single-domain (VHH) antibodies offer many advantages for high-resolution imaging, including higher density labeling and better sample penetration, and are being used for novel research applications such as live-cell STED11.
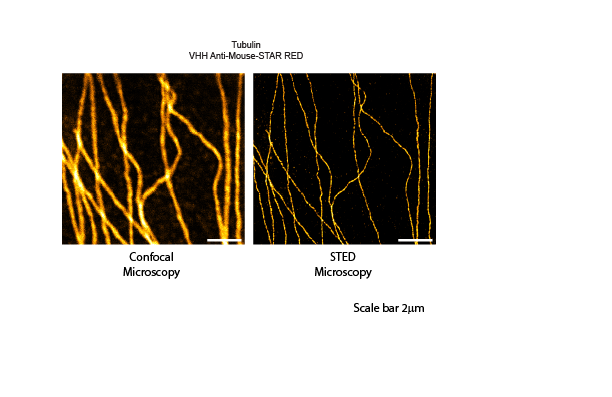
Figure 2: Single color microscopy. Indirect immunostaining was performed with JIR AffiniPure VHH™ Anti-Mouse antibodies conjugated to Abberior STAR RED. Clear improvements in resolution can be seen when comparing confocal to STED microscopy.
Supporting super-resolution microscopy
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications. We offer an extensive selection of secondary antibodies that are conjugated to fluorophores, including dyes that have been literature-cited for super-resolution microscopy, as well as our range of AffiniPure-VHH™ secondaries, which are now available from Abberior conjugated to their super bright STAR dyes making them an excellent choice for SRM.
Learn more about VHH fragment antibodies for super-resolution microscopy here
- Valli J, Garcia-Burgos A, Rooney LM, Vale de Melo E Oliveira B, Duncan RR, Rickman C. Seeing beyond the limit: A guide to choosing the right super-resolution microscopy technique. J Biol Chem. 2021;297(1):100791. doi:10.1016/j.jbc.2021.100791/
- Galbraith CG, Galbraith JA. Super-resolution microscopy at a glance. J Cell Sci. 2011;124(Pt 10):1607-1611. doi:10.1242/jcs.080085/
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19(11):780-782. doi:10.1364/ol.19.000780/
- Hell, S.W., Kroug, M. Ground-state-depletion fluorscence microscopy: A concept for breaking the diffraction resolution limit. Appl. Phys. B 60, 495–497 (1995). https://doi.org/10.1007/BF01081333
- Gustafsson MG. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc. 2000;198(Pt 2):82-87. doi:10.1046/j.1365-2818.2000.00710.x
- Liu S, Hoess P, Ries J. Super-Resolution Microscopy for Structural Cell Biology. Annu Rev Biophys. 2022;51:301-326. doi:10.1146/annurev-biophys-102521-112912/
- Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642-1645. doi:10.1126/science.1127344
- Bates M, Jones SA, Zhuang X. Stochastic optical reconstruction microscopy (STORM): a method for superresolution fluorescence imaging. Cold Spring Harb Protoc. 2013;2013(6):498-520. Published 2013 Jun 1. doi:10.1101/pdb.top075143
- Heilemann M, van de Linde S, Schüttpelz M, et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed Engl. 2008;47(33):6172-6176. doi:10.1002/anie.200802376
- Hassdenteufel S, Schuldiner M. Show your true color: Mammalian cell surface staining for tracking cellular identity in multiplexing and beyond. Curr Opin Chem Biol. 2022;66:102102. doi:10.1016/j.cbpa.2021.102102
- Schneider AFL, Benz LS, Lehmann M, Hackenberger CPR. Cell-Permeable Nanobodies Allow Dual-Color Super-Resolution Microscopy in Untransfected Living Cells. Angew Chem Int Ed Engl. 2021;60(40):22075-22080. doi:10.1002/anie.202103068
- Huang, B., Bates, M., & Zhuang, X. (2009). Super-resolution fluorescence microscopy. Annual Review of Biochemistry, 78 , 993–1016.
| Learn more: | Do more: |
|---|---|
| Indirect and direct Western blotting | Exhibition Schedule |
| Chemiluminescence western blotting | Cite and Win |
| An Introduction to Expansion Microscopy | Past Competition Winners |