"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Green fluorescent protein (GFP) was isolated from the bioluminescent jellyfish Aequorea victoria by Osamu Shimomura in 1962. It wasn't until 1994 that Martin Chalfie first applied it to monitor gene expression. The utility of GFP as a tool soon became apparent, and a raft of other useful fluorescent proteins in a rainbow of colors were isolated and developed. The value of GFP was later recognized in the 2008 Nobel Prize. Fluorescent proteins are invaluable in many aspects of life science. For example, their fusions or chimeras enable the detection and quantification of protein or gene expression, as well as the characterization of molecular interactions. Jackson ImmunoResearch now offers polyclonal Anti-GFP antibody conjugates for the detection of GFP and its derivatives across a range of applications.
GFP is a common protein tag used to generate fusion proteins in various prokaryotic and eukaryotic systems. It can be expressed either transiently or constitutively, depending on the researcher's goal. This tag is useful as a reporter molecule because it does not require exogenous substrates or cofactors to generate fluorescence. GFP is employed in numerous applications, including quantification of gene expression, protein localization within a living organism, studying protein interactions, and as a biosensor.
Developed by the laboratories of Thastrup and Falkow in the late 1990s, enhanced GFP (EGFP) possesses a single point mutation (F64L) that improves the brightness and subsequent tractability of the molecule. EGFP is the most prevalent variant of GFP used in molecular biology currently. Other variants of the initial GFP have subsequently been developed, with different spectral characteristics, resulting in yellow and blue (cyan) fluorescent proteins.
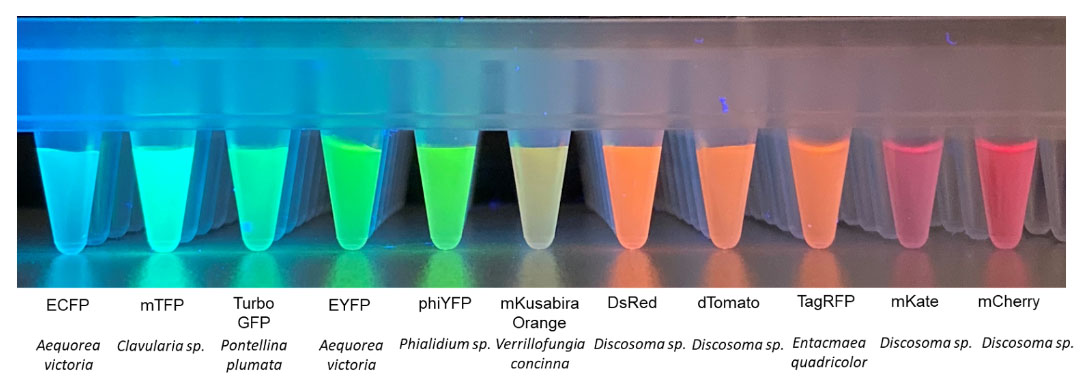
After Shimomura's discovery of GFP, scientists began to investigate the properties of fluorescent proteins from other organisms. These genetically encoded fluorescent reporters were also subject to modification to alter their spectral characteristics, allowing them to excite and emit at different wavelengths, as shown in Fig. 1.
Anti-GFP antibodies allow the detection of the GFP protein when direct fluorescence detection is not possible. Examples include autofluorescence in the GFP channel, when another reporter molecule is required (such as in Western blotting), or when the fluorescence of the native GFP is compromised or insufficient and needs to be amplified.
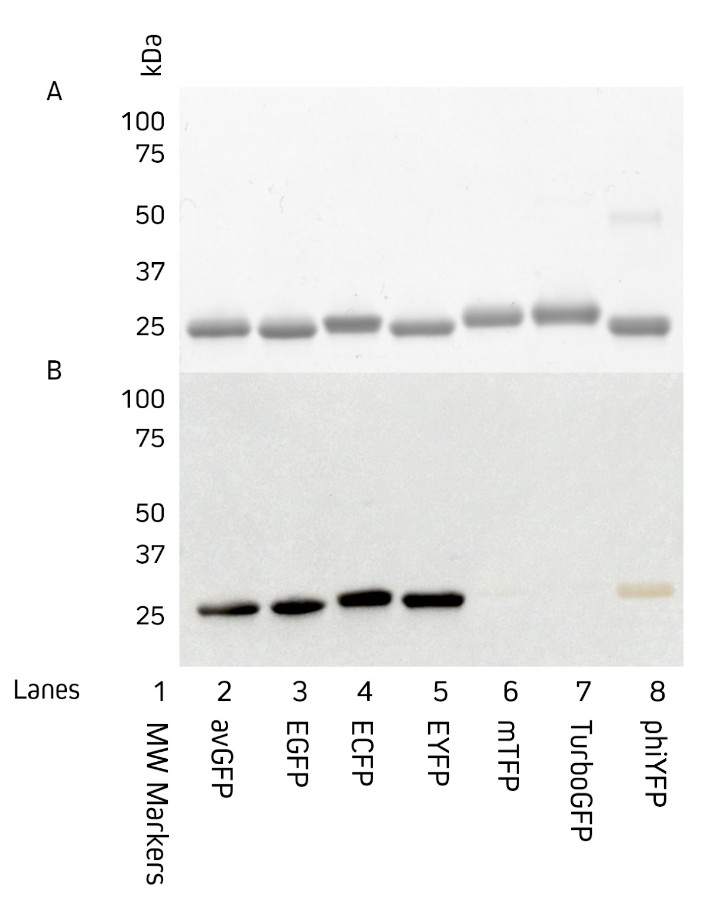
Jackson ImmunoResearch AffiniPure® Anti-GFP antibodies are available as either an affinity-purified polyclonal antibody raised in rabbit, or as a polyclonal VHH fragment from alpaca. They both can be used to detect Aequorea victoria Green Fluorescent Protein (avGFP) and its derivatives, including EGFP, ECFP, and EYFP. Figures 2 and 3 show the detection of various fluorescent proteins by AffiniPure® Rabbit Anti-GFP by western blot (Figure 2) and AffiniPure-VHH® Fragment Alpaca Anti-GFP by ELISA (Figure 3).