
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Green fluorescent protein (GFP) and its derivatives are commonly used tags in fusion proteins. They enable visualization and characterization of protein localization and interactions using a variety of imaging techniques. Read more to find out how Jackson ImmunoResearch's NEW AffiniPure® Rabbit Anti-GFP, can be used for both immunofluorescence microscopy and chromogenic IHC.
Green Fluorescent Protein (GFP) is a commonly used reporter protein that has useful characteristics when it is used to create a fusion protein, for example, visualizing protein localization, detecting and quantifying expression of transgenic proteins in vivo, and for characterizing physiological processes. GFP is maximally excited at 488 nm, which corresponds to the FITC laser line, and is optimally detected at 510 nm, making it detectable with typical lab equipment. An improved version of GFP is EGFP (Enhanced Green Fluorescent Protein), which is regularly used in fusion proteins as both an expression marker and a reporter molecule. Although GFP and its descendants are robust and tractable molecules, the effects of sample processing for microscopy techniques can damage or render it unsuitable under certain conditions. Anti-GFP antibodies allow researchers to continue to use GFP protein fusions and bypass their limitations when conditions lead to their suboptimal performance.
Like many proteins, GFP requires the conditions around it to be correct in order to be optimally functional. For GFP to fluoresce, it must form a fully folded beta-barrel structure, followed by an intramolecular reaction that requires oxygen to generate the active chromophore (Tsien, 1998). Consequently, GFP is non-fluorescent under anaerobic conditions such as those induced by FFPE (Chia et al., 2019). FFPE and other fixing techniques are often used to prevent the migration of proteins, which can prevent accurate protein localization (Chalfie and Kain., 2005; Morris et al., 2010). However, the process can functionally alter the GFP protein, weakening and destroying the protein's ability to fluoresce (Kusser et al., 2003).
Another limitation of GFP that prevents its functionality is its sensitivity to acid, which may preclude its use when visualizing certain cellular organelles (Shinoda et al., 2018). GFP's chromophore can only absorb and emit light in its protonated state (Tsien, 1998). Therefore, at pHs below its pKa (<pH 6.0), where the chromophore is present in its deprotonated state, the efficiency of the protein to fluoresce is reduced.
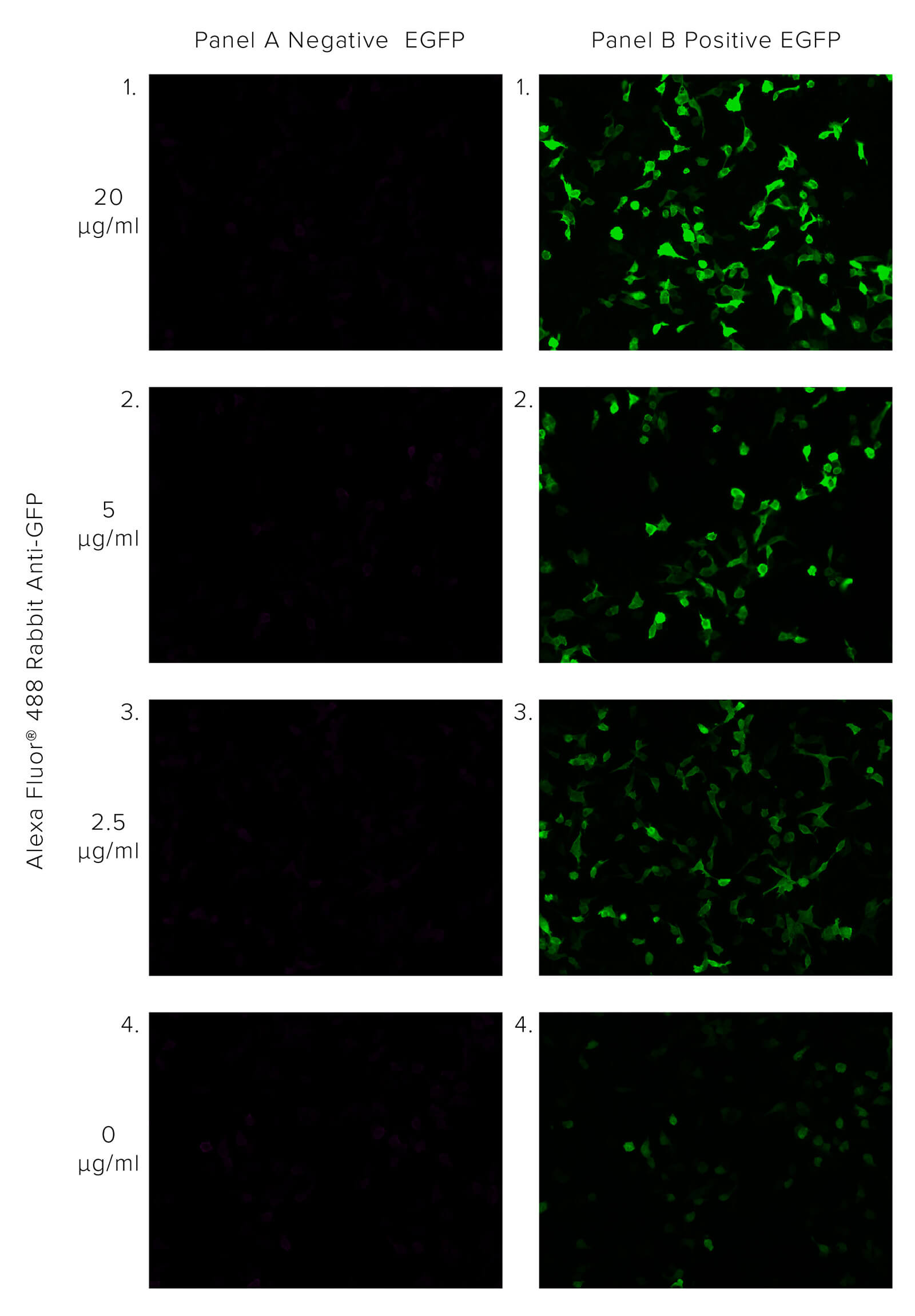
JIR Anti-GFP antibodies offer robust detection of GFP, recognizing GFP in native or fixed/denatured conditions as long as the protein is not degraded, enabling the GFP tag to be used in circumstances where the protein would not be functional. The polyclonal format of JIR Rabbit Anti-GFP enables the binding of multiple antibodies to the target, bringing additional fluorescent molecules, thus enhancing the GFP signal beyond the output associated with GFP alone. This is demonstrated in Figure 1, where HEK 293T cells transfected with EGFP are probed with Alexa Fluor® 488 Rabbit Anti-GFP. We can observe the amplification of the GFP signal as the concentration of Rabbit Anti-GFP increases.
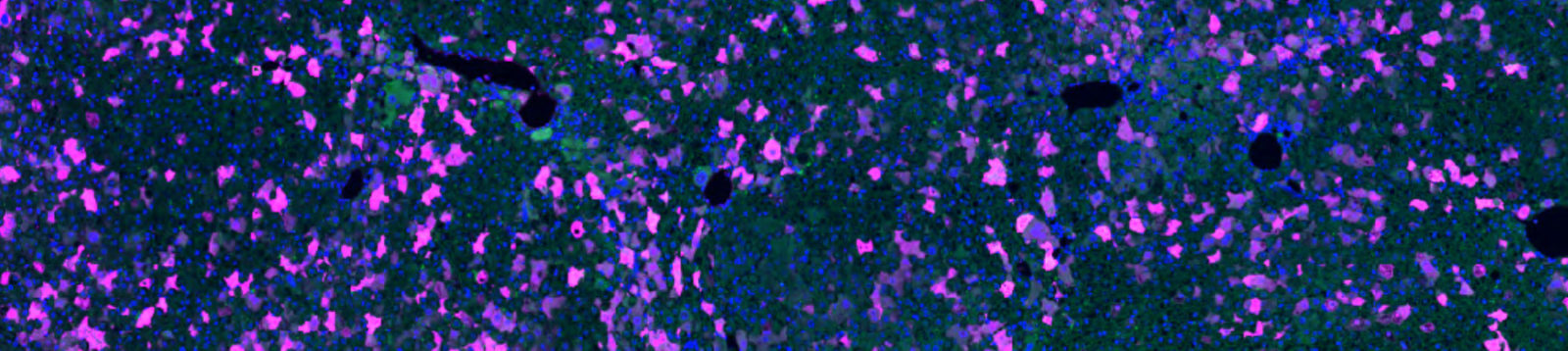
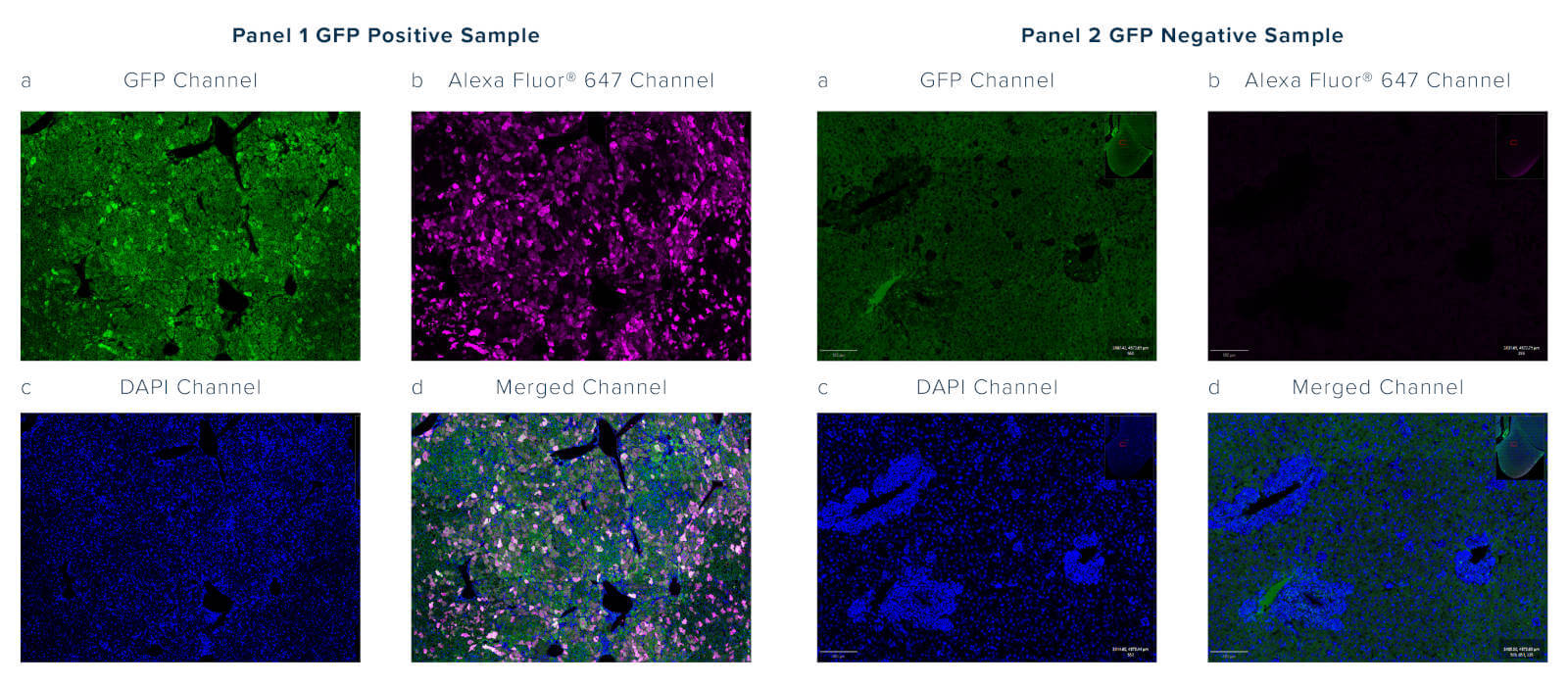
Autofluorescence is intrinsic fluorescence that occurs due to cellular components or as an artifact of sample processing that generates background signal. It often interferes with the detection and interpretation of genuine signals from target proteins. Autofluorescence may occur across the spectrum but is often observed emitting between 350 and 550 nm, which overlaps with the emission profile of GFP; this can be problematic as the GFP signal cannot be distinguished over the background autofluorescence (figure 2, panel 1a).
Anti-GFP antibodies can be chosen as conjugates to a wide range of fluorophores, enabling researchers to choose an emission profile that does not overlap with the GFP signal or sample autofluorescence.
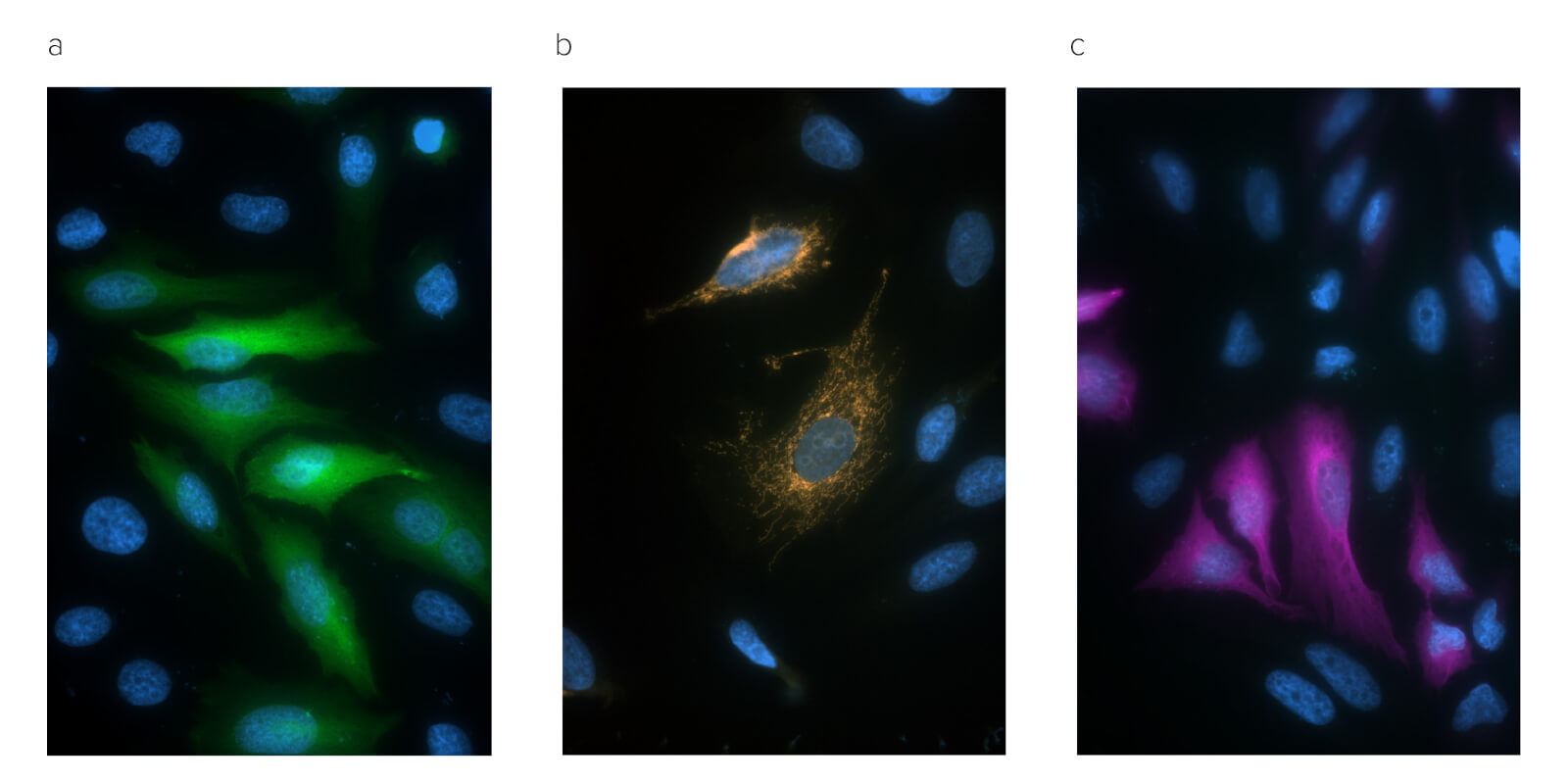
Modern microscopy techniques continue to evolve, often seeking to label multiple targets simultaneously or push resolution boundaries.
Jackson Immunoresearch Anti-GFP is available in a wide range of fluorescent conjugates, providing different emission profiles, so that conjugates can be appropriately selected for the available equipment and requirements of the experiment. Conjugated antibodies may also be chosen to increase panel flexibility, so more targets can be accommodated in a single experiment. When fluors are selected in longer wavelengths, such as Alexa Fluor® 647, signals can be resolved without spectral unmixing, which often requires additional or expensive equipment or data compensation.
Conjugated Anti-GFP antibodies can also add versatility to experiments restricted by equipment limitations, such as when fluorescent imaging systems are unavailable or unsuitable. Jackson ImmunoResearch Anti-GFP antibody is available conjugated to reporter enzymes, Horseradish peroxidase (HRP), and Alkaline phosphatase, as well as a biotinylated conjugate facilitating the use of chromogenic IHC staining techniques and amplification protocols such as ABC, LSAB.
Transgenic animal models are powerful tools for gaining deeper knowledge about protein expression and interactions within an organism. Transproteins are regularly generated as fusions with reporter molecules such as GFP to enable their detection, co-localization, or characterize their interactions using such techniques as FRET (Förster resonance energy transfer) or SRM (Super-resolution microscopy). Chromogenic IHC is an immunohistochemical technique that uses antibodies to target protein antigens, followed by a chromogen/substrate combination to visualize them in tissue samples. Here, we investigate the presence of a constitutively expressed GFP fusion protein in the liver of a transgenic mouse, using direct and indirect immunostaining methods incorporating Jackson ImmunoResearch Rabbit Anti-GFP antibody conjugates.
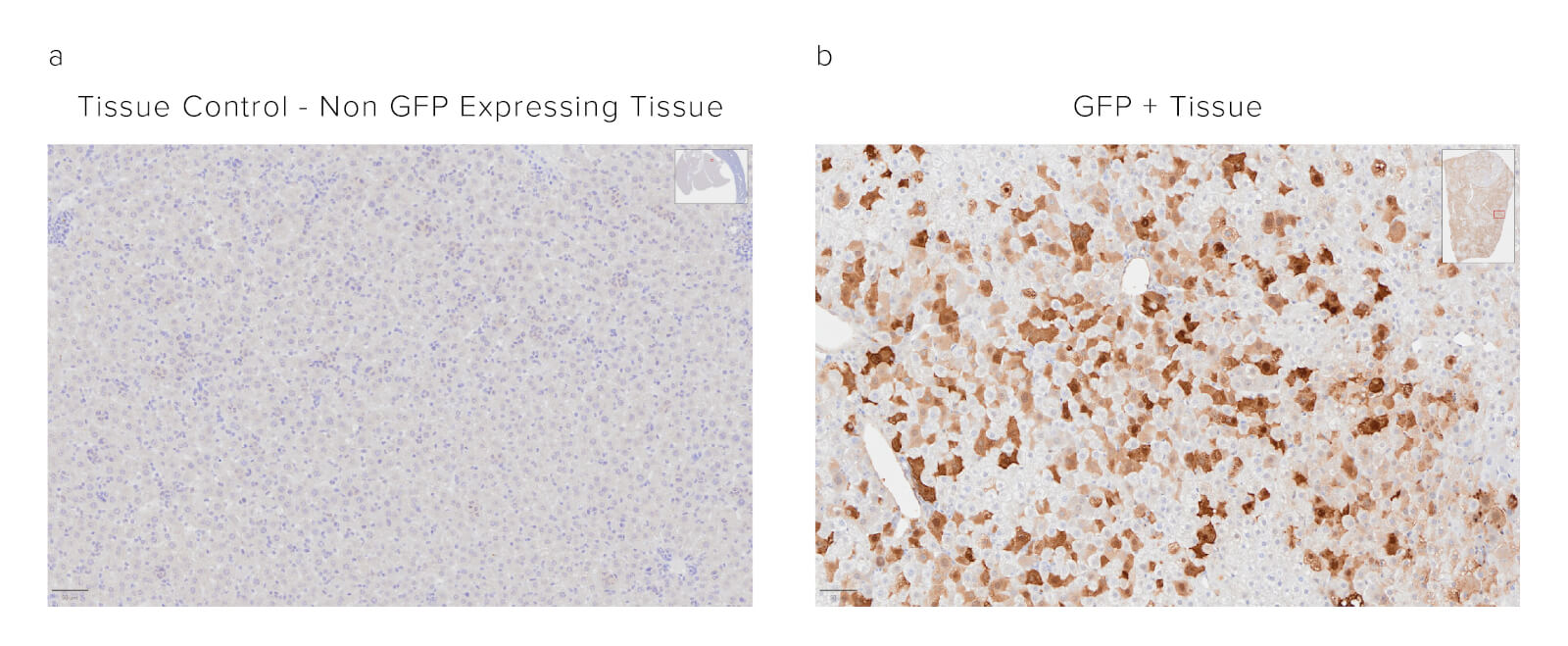
Formalin-fixed paraffin-embedded (FFPE) mouse liver. The sample was baked, deparaffinized, and hydrated before Heat-Induced Epitope Retrieval (HIER) at 120/90°C, pH 6. Endogenous peroxidase was inactivated with 3% Hydrogen peroxide in dH2O before blocking in 3% BSA in PBS for 1 hr. The sample was incubated with Peroxidase Rabbit Anti-GFP (300-035-245) at a dilution of 1:1000. The sample was washed and incubated with a DAB substrate and imaged at 20x by the Histology Research Core Facility in the Dept. of Cell and Molecular Physiology at University of North Carolina.
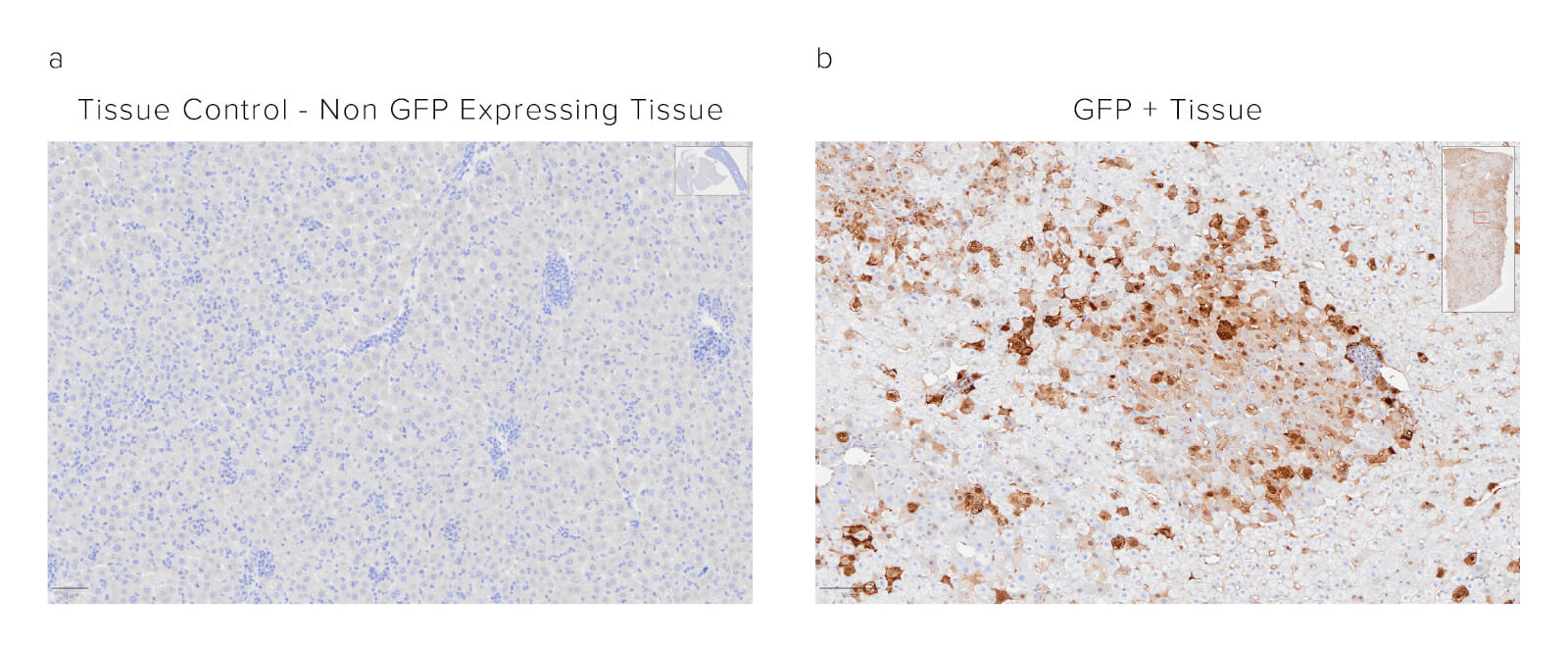
Sample: Formalin-fixed paraffin-embedded (FFPE) mouse liver. The sample was baked, deparaffinized, and hydrated before HIER at 120/90°C pH 6. Endogenous peroxidase was inactivated with 3% Hydrogen peroxide in dH2O before blocking in 3% BSA in PBS for 1hr. Sample was then incubated with Biotinylated Rabbit Anti-GFP (300-065-245) at a dilution of 1:1000. Sample was washed and then incubated with ABC kit at 1:50 dilution (Vector) and imaged at 20x by the Histology Research Core Facility in the Dept. of Cell and Molecular Physiology at University of North Carolina.
Chromogenic IHC is a powerful tool for detecting fusion proteins in transgenic animal models. Jackson ImmunoResearch Rabbit Anti-GFP antibody conjugates offer specific and sensitive detection of GFP in FFPE tissue when used with standard chromogenic substrate protocols, or ABC signal amplification methods.