
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Fab fragment antibodies are generated by papain digestion of whole IgG antibodies to remove the entire Fc fragment, including the hinge region. These antibodies are monovalent, containing only a single antigen binding site. The molecular weight a Fab fragment is about 50 kDa. They can be used to block endogenous immunoglobulins on cells, tissues or other surfaces, and to block the exposed immunoglobulins in multiple labeling experiments using primary antibodies from the same species.
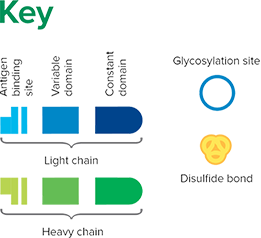
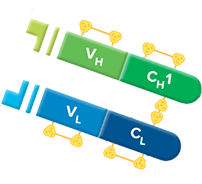
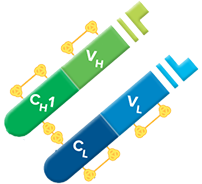
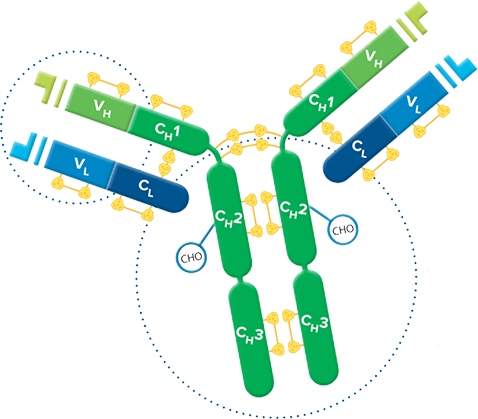
Monovalent Fab fragments of affinity-purified secondary antibodies are offered to block endogenous immunoglobulins in tissue sections or on cell surfaces, to cover (block) immunoglobulins when double labeling primary antibodies from the same host species, or to Fab-label primary antibodies prior to incubation with the experimental sample. They can be used for these purposes because each Fab fragment has only a single antigen binding site (i.e. they are monovalent), and they are non-precipitating.
Divalent (whole IgG or F(ab')2 fragment) antibodies are not recommended for blocking because they have two antigen binding sites. After binding endogenous IgG or the first primary antibody, some antigen binding sites on a divalent secondary antibody may remain unoccupied, which could capture a primary antibody introduced in a subsequent step. Capture of the primary antibody would allow detection of that primary by a labeled secondary antibody, resulting in background staining or apparent signal overlap. The use of monovalent Fab fragments avoids this problem.