Journal club: Affinity maturation of neutralizing antibodies improves in vivo protection against SARS-CoV-2.

Since the start of the COVID-19 pandemic, multiple vaccines have been developed that protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The way in which these work is still being deduced, but several studies have shown that the elicitation of neutralizing antibodies (nAbs) has a central role in combating infection. Recombinantly produced nAbs promise to supplement protection in populations that respond poorly to vaccines, including immunocompromised individuals. However, a major challenge when developing recombinant nAbs lies in overcoming viral escape mutations. By using directed evolution to engineer three previously characterized nAbs isolated from convalescent COVID-19 patients, researchers at The Scripps Research Institute in La Jolla, CA, were able to improve protection in a Syrian hamster model compared to using the parental antibodies. Their data suggest that appropriate affinity maturation of nAbs is an effective strategy to resist viral variation.

Engineering higher affinity SARS-CoV-2 antibodies
One of the main ways in which SARS-CoV-2 infects airway epithelial cells is through binding via its spike proteins to angiotensin-converting enzyme 2 (ACE2), which is expressed at the epithelial cell surface. Neutralizing antibodies (nAbs) serve to block this interaction and prevent viral uptake. However, SARS-CoV-2 can escape these effects by developing spike protein mutations that disrupt nAb binding. To determine whether the protective efficacy of existing nAbs could be enhanced through engineering, Zhao et al. selected three nAbs known to impede SARS-CoV-2 binding to ACE2 (CC12.1, CC6.30 and CC6.33) and subjected them to rapid affinity maturation using a platform termed SAMPLER. Briefly, this involved synthesizing heavy chain and light chain libraries with up to three mutations per chain for display on the surface of yeast, followed by panning for high-affinity clones; the antibody sequences were then recovered, and twelve improved variants of each antibody were reformatted and expressed as IgGs for characterization.
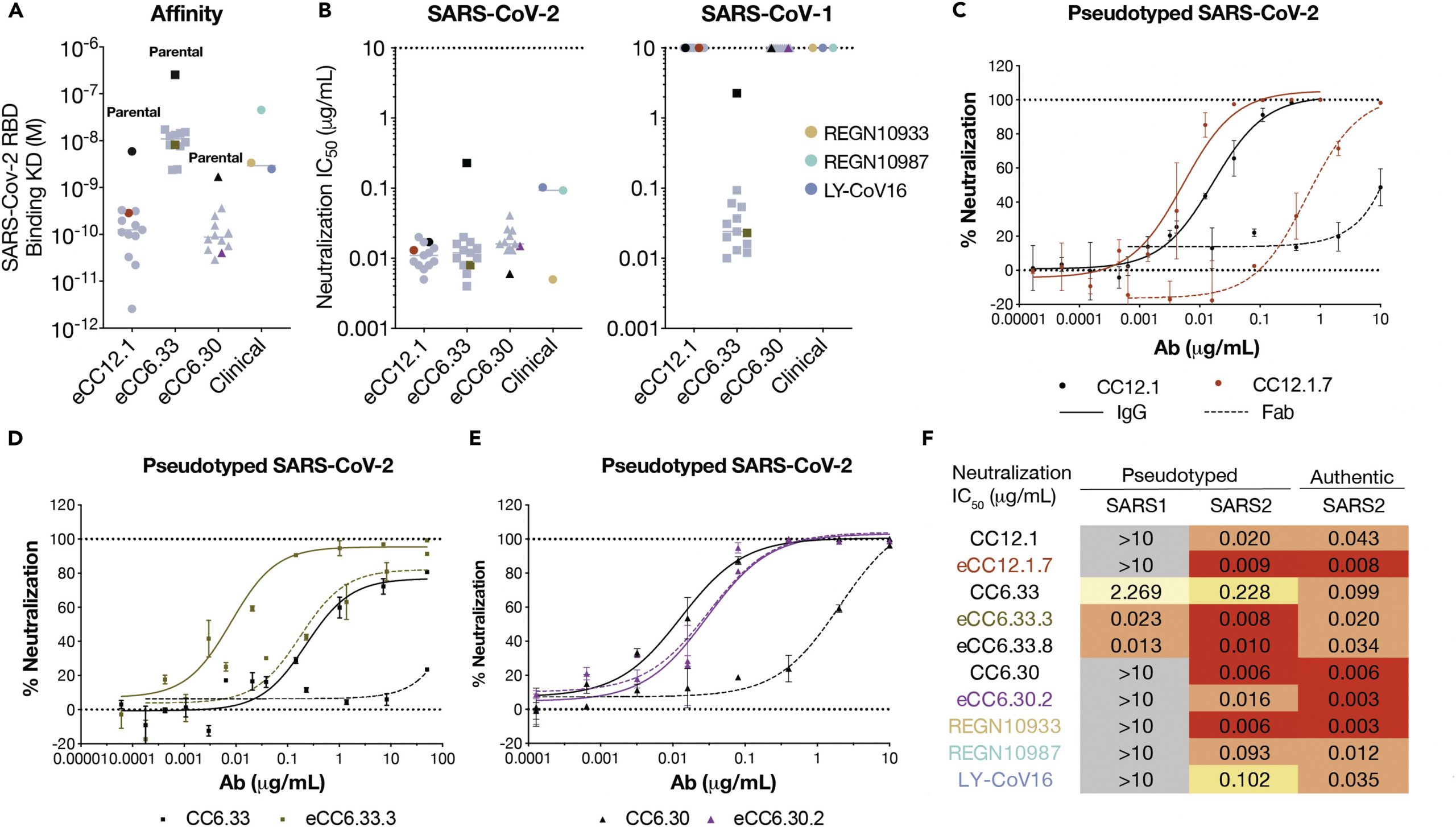
(A) Enhanced and parental nAbs binding affinity against SARS-CoV-2 RBD by surface plasmon resonance. Parental nAbs are highlighted in black. eCC12.1.7, eCC6.33.3 and eCC6.30.2 are highlighted, whereas other engineered variants are colored in grey. RBD binding to antibodies via an Fc-capture, multi-cycle method. Association and dissociation rate constants were calculated through a 1:1 Langmuir binding model using the BIA evaluation software. (B) Neutralization IC50 against pseudotyped SARS-CoV-2 and SARS-CoV-1 viruses. (C–E) SARS-CoV-2 pseudovirus neutralization curves of (C) parental CC12.1 and eCC12.1.7, (D) parental CC6.33 and eCC6.33.3, (E) parental CC6.30 and eCC6.30.2 in IgG and Fab molecules. Solid lines represent IgG neutralization whereas dashed lines represent Fab neutralization. Data are represented as mean ± SD. Data are representative of at least two independent experiments. (F) Summary table of nAb neutralization IC50 against pseudotyped SARS-CoV-1 and SARS-CoV2, as well as replicating SARS-CoV-2. (Figure taken from Zhao et al., 2022)
Evaluation of neutralizing potency
To assess the neutralizing potency of the affinity-matured nAbs, Zhao et al. seeded Vero E6 cells into 96 well plates and added various premixed solutions of nAbs/SARS-CoV-2 variants of interest. The viral variants included B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.1 (Kappa), and B.1.617.2 (Delta), as well as variants featuring single point mutations in the receptor binding domain. After incubating to allow for viral transduction, the cells were fixed, permeabilized, and blocked, and a mixture of human anti-SARS-CoV-2 primary antibodies was added. A peroxidase-conjugated goat anti-human IgG secondary antibody (Jackson ImmunoResearch 109-035-088) and a chemiluminescent HRP substrate were then used to detect viral uptake. Key findings included the observation that most engineered forms of CC12.1 were able to neutralize the gamma and beta lineages, which completely escaped the parental nAb and the discovery that an engineered form of CC6.30.2 was functional against viral strains containing the L452R and E484Q mutations, while the parental nAb was ineffective.
Assessment of in vivo protectivity
The in vivo efficacy of the engineered nAbs was evaluated using a Syrian hamster model of COVID-19 infection. 72 hours after infusing groups of animals with serially diluted doses of different nAbs, Zhao et al. administered an intranasal challenge with SARS-CoV-2 and performed a range of measurements. These included monitoring daily for weight loss as an indicator of disease, collecting sera at days 0 and 7 to evaluate antibody half-lives, and harvesting lung tissue to quantify viral titers via a plaque assay. The resultant data showed an engineered form of CC12.1 (eCC12.1.6) to exhibit a dose-dependent protective response both in terms of weight loss and lung viral titers, while the parental nAb provided no protection compared to a control group. An engineered version of CC6.33 (eCC6.33.3) was also found to be superior at controlling lung viral load compared to the original nAb.
Conclusion
The data generated at The Scripps Research Institute suggest affinity maturation to be a viable strategy for protecting against emerging viral variants. In further support of this, Zhao et al. have recently shown eCC12.1.6 to have significant neutralizing activity against the Omicron variant, a highly mutated form of SARS-CoV-2 which was first reported at the time of this study. Critically, reducing viral escape pathways using methods such as affinity maturation promises to help limit the spread of COVID-19 and may also have utility for tackling other viral diseases.
Featured products:
- Peroxidase AffiniPure Goat Anti-Human IgG H+L (Catalog# 109-035-088)
- Alkaline Phosphatase AffiniPure Goat Anti-Human IgG, Fcγ fragment specific (Catalog# 109-055-008)
Article reference:
- Zhao F, Keating C, Ozorowski G, et al. Engineering SARS-CoV-2 neutralizing antibodies for increased potency and reduced viral escape pathways. iScience. 2022;25(9):104914. doi:10.1016/j.isci.2022.104914


