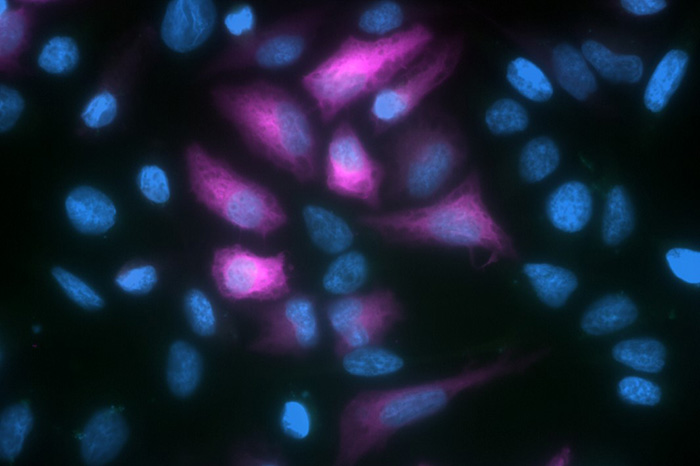
Since its discovery in the 1960s, Green Fluorescent Protein (GFP) has enabled researchers to investigate an ever-increasing number of biological phenomena. Examples include its use for studying protein localization, gene expression, and cell fate, as well as chromosome replication, intracellular transport, and organelle inheritance. Here, we look at some common applications of GFP and explain how anti-GFP antibodies can augment these types of studies.
What is GFP?
GFP is a 27 kDa protein that was discovered in the jellyfish Aequorea victoria1. It contains a tripeptide motif (Ser65-Tyr66-Gly67), which spontaneously forms a chromophore when the protein folds into its native conformation, surrounded by a β-barrel, which protects against fluorescent quenching. GFP has major and minor excitation peaks at wavelengths of 395 and 475 nm, respectively, and a sharp emission peak at 505 nm, which produces the characteristic green fluorescence. The spectral properties, brightness, and photostability of GFP can be altered by introducing specific point mutations. For example, the S65T mutation yields a single excitation peak at 488 nm and the Y66H mutation produces a derivative that emits in the blue region of the visible spectrum, while the S65T/F64L double mutant has a 30-fold higher fluorescent intensity than the wild-type protein2-4.
Learn about the discovery of GFP in our previous blog.
Common GFP applications
Because GFP has an integral chromophore that requires nothing but molecular oxygen for its maturation, it lends itself to a broad range of research applications. The following are some common examples:
Fusion tagging
GFP fusion proteins are formed by linking the GFP gene to the gene encoding the protein of interest, such that both genes are transcribed and translated as a single unit. A simple way of achieving this is to clone a cDNA for the target protein into a commercial GFP vector. The modified DNA can then be introduced into cells via transient or stable transfection, where protein expression is tracked by monitoring the fluorescent signal.
Studying gene regulation
GFP has been widely used as a reporter gene to investigate how gene expression is regulated. In this setting, an expression construct is generated that contains the gene regulatory elements to be analyzed, as well as the sequences required for transcription of functional mRNA, and that substitutes the GFP gene for the gene of interest. Following introduction of the construct into cells, GFP fluorescence can be correlated to gene expression.
Evaluating transfection efficiency
Besides enabling researchers to study gene regulation, using GFP as a reporter gene allows for evaluating transfection efficiency. However, the main difference between the two approaches is that evaluating transfection efficiency usually involves placing the GFP gene under the control of its own constitutive promoter. This is to account for the fact that the gene of interest might only be expressed under certain conditions. Importantly, using GFP for evaluating transfection efficiency allows for normalization between different experiments.
Förster resonance energy transfer (FRET)
FRET occurs when two fluorescent molecules (a donor and an acceptor) are in close proximity to one another. Following excitation of the donor, energy is transferred to the acceptor, causing it to emit a detectable signal. Critically, the emission spectrum of the donor must overlap with the excitation spectrum of the acceptor. GFP and its derivatives are widely used for FRET-based applications, where they can serve as either donors or acceptors, depending on the experimental requirements.
Split GFP applications
Many proteins, including GFP, can be split into fragments that spontaneously reassemble into a functional biomolecule5. This property has been widely exploited for bimolecular fluorescence complementation (BiFC), a technique that detects protein-protein interactions by tethering each of the fragments to a potential binding partner6. BiFC was first demonstrated for GFP in 2000,7. However, the methodology has since evolved into techniques including tripartite split-GFP complementation, which minimizes protein interference and aggregation, and Fluorescent Assembly of Split-GFP for Translation Tests (FAST), which has utility for monitoring cell-free protein synthesis8,9.
Fluorescence microscopy
One of the best-known applications of GFP is its use for microscopy-based research. When working with living cells that have been transfected with a GFP construct, fluorescence can be measured directly. However, when working with fixed samples, the fluorescent signal can sometimes be compromised by sample processing. One way of addressing this problem is to use an anti-GFP antibody for detection. This can either be labeled with a fluorophore or detected with an appropriate secondary antibody, which offers the advantage of signal amplification.
Fluorescence-activated cell sorting (FACS)
FACS is a type of flow cytometry that isolates specific cell types from a heterogeneous population based on light scatter and fluorescence. Provided the FACS instrument is configured for GFP detection, with a 488 nm laser and a 510 nm detector, it can be used to separate cells that express GFP from those that do not.
Protein purification
GFP can be used for protein purification with an anti-GFP antibody, typically through attaching the antibody to functionalized agarose or magnetic beads. Available options include Protein A and Protein G beads, which should be matched to the antibody species, class, and sub-class to ensure optimal binding, and beads functionalized with secondary antibodies, which should be paired with the host species of the anti-GFP antibody.
Supporting your research
Jackson ImmunoResearch offers a growing selection of primary antibodies targeting popular antibody tags. Our anti-GFP rabbit polyclonal is available unconjugated or with a choice of 11 different labels, including R-Phycoerythrin (RPE) and various Alexa Fluor® dyes, and reacts with Aequorea victoria GFP, as well as derivatives such as EGFP, ECFP, and EYFP.
References
- Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223-239. doi:10.1002/jcp.1030590302 https://pubmed.ncbi.nlm.nih.gov/13911999/
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373(6516):663-664. doi:10.1038/373663b0 https://pubmed.ncbi.nlm.nih.gov/7854443/
- Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91(26):12501-12504. doi:10.1073/pnas.91.26.12501 https://pubmed.ncbi.nlm.nih.gov/7809066/
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene. 1996;173(1 Spec No):33-38. doi:10.1016/0378-1119(95)00685-0 https://pubmed.ncbi.nlm.nih.gov/8707053/
- Romei MG, Boxer SG. Split Green Fluorescent Proteins: Scope, Limitations, and Outlook. Annu Rev Biophys. 2019;48:19-44. doi:10.1146/annurev-biophys-051013-022846 https://pubmed.ncbi.nlm.nih.gov/30786230/
- Kodama Y, Hu CD. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. Biotechniques. 2012;53(5):285-298. doi:10.2144/000113943 https://pubmed.ncbi.nlm.nih.gov/23148879/
- Ghosh I, Hamilton AD, Regan L. Antiparallel Leucine Zipper-Directed Protein Reassembly: Application to the Green Fluorescent Protein. J Am Chem Soc. 2000;122:5658-5659. DOI:10.1021/JA994421W https://www.semanticscholar.org/paper/Antiparallel-Leucine-Zipper-Directed-Protein-to-the-Ghosh-Hamilton/84e65b5eb57f93deadee8983bd67315c1a980561
- Cabantous S, Nguyen HB, Pedelacq JD, et al. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep. 2013;3:2854. doi:10.1038/srep02854 https://pubmed.ncbi.nlm.nih.gov/24092409/
- Pham, TD, Poletti, C, Tientcheu, TMN et al. FAST, a method based on split-GFP for the detection in solution of proteins synthesized in cell-free expression systems. Sci Rep. 2024;14:8042. https://doi.org/10.1038/s41598-024-58588-5