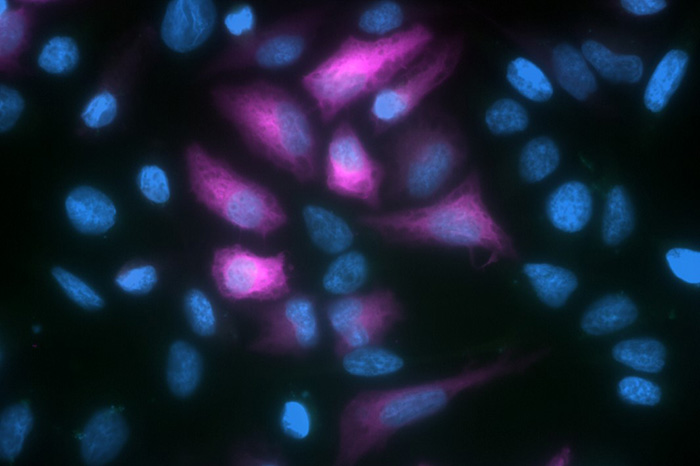
Green fluorescent protein, more commonly known as GFP, was discovered in 1962. Since then, it has become one of the most widely exploited proteins for scientific research and has driven the development of many different microscopy-based applications. A limitation of GFP is that its fluorescence can be weakened or lost under certain fixation conditions. However, this problem can be resolved by using anti-GFP antibodies for signal enhancement.
A Brief History of GFP
GFP was discovered by Osamu Shimomura in 1962 while investigating the natural bioluminescence of the jellyfish Aequorea victoria1. The aim of the original study was to identify the source of the blue light emitted by these organisms, which Shimomura found to be the Ca2+ sensitive protein aequorin. However, during the purification of aequorin, a second protein that exhibited a bright green fluorescence was also isolated, albeit in only trace amounts.
In 1974, the green fluorescence of this second protein was shown to occur as a result of Förster resonance energy transfer (FRET) from aequorin2. But it was not until 1979 that the real breakthrough came, when Shimomura found the GFP chromophore to be part of the peptide chain, opening up the possibility of cloning the protein3. GFP was successfully cloned and sequenced by Prasher et al. in 19924. Then, just two years later, Chalfie et al. expressed GFP in Escherichia coli and Caenorhabditis elegans5.
The structure of GFP was resolved by Tsien et al. in 1996, who showed the wild-type protein to consist of 238 amino acids (26.9kDa), folded to produce an α-helix surrounded by a β-barrel6. The chromophore, located within the α-helix (and thus protected by the β-barrel from fluorescent quenchers such as water, triplet oxygen, and photoisomerization), was found to be formed from serine 65, tyrosine 66, and glycine 67. GFP has major and minor excitation peaks at wavelengths of 395 nm and 475 nm, respectively, and emits fluorescence at 508nm, but only when the protein is correctly folded.
Tsien et al. subsequently engineered native GFP to be brighter and more photostable, as well as developed various GFP derivatives with different spectral characteristics7. Examples of these newer fluorescent proteins include enhanced GFP (eGFP), and cyan, blue, and yellow fluorescent proteins (CFP, BFP, and YFP, respectively). Shimomura, Chalfie, and Tsien were jointly awarded the Nobel Prize for Chemistry in 2008 for their discovery and development of the green fluorescent protein.
GFP applications
GFP has been widely adopted for microscopy-based research as it enables the visualization of proteins of interest with no need for exogenous substrates or co-factors. The most common approach involves cloning the GFP gene downstream of a target gene in a cell line or model organism, whereby expression of the resultant fusion protein allows for monitoring target localization in either fixed or living samples.
A limitation of this method when working with fixed samples is that the fluorescence can be compromised by certain fixation conditions. One way of addressing this issue is to use anti-GFP antibodies to detect the expressed fusion protein. These can be either directly labeled with another fluorophore or paired with an appropriate secondary antibody for detection. An advantage of using secondary antibodies is that they can provide signal amplification.
For live-cell imaging applications, the time taken for GFP maturation prohibits the analysis of short-lived proteins, such as transcription factors, which are often degraded before the GFP produces a fluorescent signal. This problem is being overcome with the development of genetically encoded antibody-based probes such as antigen-binding fragments (Fabs), single-chain variable fragments (scFvs), and camelid nanobodies that are pre-labeled with mature GFP or other fluorophores8,9.
Another important application for GFP is its use for co-localization studies. Here, one protein is labeled with GFP while another is labeled with a different fluorophore such that the emission spectrum of one of the fluorophores overlaps with the excitation spectrum of the other. By illuminating the sample with a laser that excites just the first fluorophore, a signal will only be produced if the two proteins are in close enough proximity for FRET to take place.
GFP has also been exploited for studies based on fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP). These techniques involve photobleaching a region of interest within a cell, often an area of the cell membrane, before monitoring the diffusion of unbleached GFP back into the area in question (FRAP) or tracking the decrease in fluorescence outside of the region of interest as the bleached proteins disseminate (FLIP).
Supporting your research
GFP and its derivatives represent indispensable tools for scientific research that underpin both established and emerging applications. Jackson ImmunoResearch has recently launched a new anti-GFP antibody, which is available conjugated to a range of conjugates, including Alexa Fluor® dyes, or they can be paired with our high-quality anti-rabbit secondaries to amplify GFP detection.
References:
- SHIMOMURA O, JOHNSON FH, SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223-239. doi:10.1002/jcp.1030590302 https://pubmed.ncbi.nlm.nih.gov/13911999/
- Shimomura O, Johnson FH, Morise H. Mechanism of the luminescent intramolecular reaction of aequorin. Biochemistry. 1974;13(16):3278-3286. doi:10.1021/bi00713a016 https://pubmed.ncbi.nlm.nih.gov/4152180/
- O. Shimomura, Structure of the chromophore of Aequorea green fluorescent protein, FEBS Letters, Volume 104, Issue 2, 1979, Pages 220-222, https://www.sciencedirect.com/science/article/pii/0014579379808182
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111(2):229-233. doi:10.1016/0378-1119(92)90691-h https://pubmed.ncbi.nlm.nih.gov/1347277/
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802-805. doi:10.1126/science.8303295 https://pubmed.ncbi.nlm.nih.gov/8303295/
- Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273(5280):1392-1395. doi:10.1126/science.273.5280.1392 https://pubmed.ncbi.nlm.nih.gov/8703075/
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509-544. doi:10.1146/annurev.biochem.67.1.509 https://pubmed.ncbi.nlm.nih.gov/9759496/
- Lyon K, Stasevich TJ. Imaging Translational and Post-Translational Gene Regulatory Dynamics in Living Cells with Antibody-Based Probes. Trends Genet. 2017;33(5):322-335. doi:10.1016/j.tig.2017.02.003 https://pubmed.ncbi.nlm.nih.gov/28359585/
- Zhao N, Kamijo K, Fox PD, et al. A genetically encoded probe for imaging nascent and mature HA-tagged proteins in vivo. Nat Commun. 2019;10(1):2947. Published 2019 Jul 3. doi:10.1038/s41467-019-10846-1 https://pubmed.ncbi.nlm.nih.gov/31270320/