Heavy-Chain Variable (VHH) Antibodies: Diagnosis, Treatment and Prevention of Covid-19 and Novel Viral Infections
Coronaviruses are a family of enveloped, positive-sense RNA viruses affecting the upper-respiratory tract, usually causing only mild illnesses, similar to the common cold. However, three zoonotic pathogens, severe acute respiratory syndrome coronavirus (SARS-CoV)-1, Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2, that have emerged in the past two decades lead to severe and often fatal outcomes in humans.1 As of July 22nd 2020, the current coronavirus SARS-CoV-2 pandemic has infected more than 15 million people in 188 countries and the death toll has surpassed 600,000.2
This novel virus was transmitted to humans in late 2019 and as of July 2020 there are no approved therapeutic interventions available. Millions of people are being advised to stay at home to reduce the spread of SARS-CoV-2 and limit the death toll. However, to fully combat this virus, effective vaccines and treatments for this disease are essential.1
Treatment Targets for SARS-CoV-2
One promising therapeutic target is the spike (S) glycoprotein found on the surface of the outer membrane envelope of coronaviruses. This S protein forms a trimeric complex with two subunits, S1 and S2, separated by a protease cleavage site. The S1 subunit contains the receptor-binding domain (RBD), which interacts with a receptor protein on the surface of the host cell – angiotensin converting enzyme 2 (ACE2) for SARS-CoV-1 and SARS-CoV-2 and dipeptidyl peptidase 4 (DPP4) for MERS-CoV.1 The S2 subunit is then responsible for fusing the viral envelope and host membranes to allow the virus particle to enter the host cell.4
Drugs that bind to the RBD on the S1 subunit of the S protein inhibit binding to the ACE2 receptor and can prevent fusion of the virus envelope with the host cell, resulting in neutralization of the coronavirus. Interestingly, cryoelectron microscopy has demonstrated that the RBD frequently changes conformation. Two major configurations have been characterized; the up conformation, where the RBD is accessible and can readily engage with the host-cell receptor, and the down conformation, where binding is prevented as the RBD is hidden by the top of the S2 subunit. Therefore, drug binding is also thought to only be possible in the up conformation.3,4
VHH Antibodies Demonstrate Efficient Neutralizing Activity
Several RBD-specific human monoclonal antibodies derived from former patients have been isolated and reported for use against coronaviruses. They have shown strong neutralizing activities in animal models by binding with coronavirus spike protein to inhibit their abilities to infect cells. This method is used to interact with and augment the host’s immune systems which contributes to efficacy in fighting the coronavirus as it has been suggested that only 1% may have naturally high levels of antibodies that could neutralize the virus without intervention.5
These conventional antibodies are relatively large in size (~160 kDa), have poor pharmacokinetics, high production costs and are difficult to store and transport.3,4 Given these challenges, smaller biologicals like heavy-chain variable (VHH) antibodies, also referred to as nanobodies, are being investigated as alternatives to canonical antibodies because of their higher stability and ability to bind to epitopes that are inaccessible to conventional antibodies.6
Zhao et al. (2018) developed a novel VHH antibody, NbMS10, designed for the therapeutic treatment of MERS-CoV. NbMS10 bound to the RBD of the S protein with a high binding affinity (Kd, 8.71 x 10-10 M), which prevented MERS-CoV from binding to the host-cell receptor DPP4 in mice. In addition, a relatively low dose (ND50, 3.52 μg/mL) was found to be sufficient to neutralize the virus, suggesting that NbMS10 could be a potential therapeutic for MERS-CoV.4
However, due to their small size, VHH antibodies tend to have a fast-renal clearance, which may limit their applications. Researchers found that NbMS10 was cleared from the serum quickly in mice, with the binding affinity for MERS-CoV RBD completely lost 10 days post injection.
A human-Fc-fused version, NbMS10-Fc, was constructed increasing the size of the VHH antibody from 16 kDa to 50 kDa to try and overcome this issue. NbMS10-Fc demonstrated comparable binding affinity (8.71 x 10-10 and 3.46 x 10-10 M, respectively) and neutralizing activity (3.52 and 2.33 μg/mL, respectively) to NbMS10, but had a much slower clearance rate, with stable binding for MERS-CoV RBD still observed 10 days post injection.4
These data were comparable to those observed for RBD-specific conventional immunoglobulin Gs (IgG) (Kd, 7.12 x 10-10–4.47 x 10-10 M; ND50, μg/mL–ng/mL). The cross-neutralizing activity of both VHH antibodies against divergent MERS-CoV strains was also found to be high, with the ND50 ranging from 0.003–0.979 μg/mL and 0.003–0.067 μg/mL for NbMS10 and NbMS10-Fc, respectively. This research indicates VHH antibodies show promise as broad-spectrum antivirals and may be beneficial in the current SARS-CoV-2 pandemic.4
More recently, Wrapp et al. isolated the VHH antibody SARS VHH-72 from a llama immunized with prefusion-stabilized coronavirus spikes directed against SARS-CoV-1 RBD. SARS VHH-72 demonstrated nanomolar binding affinity and was capable of neutralizing pseudotyped SARS-CoV-1 viruses in vitro. These promising results led to the researchers investigating the effect of this VHH antibody on SARS-CoV-2.
Surface plasmon resonance analysis indicated SARS VHH-72 had high affinity to SARS-CoV-2 RBD, however no interaction was detected using ELISA and the VHH antibody was not able to neutralize SARS-CoV-2 pseudoviruses. Therefore, using a similar approach to Zhao et al., the group engineered SARS VHH-72 into a bivalent Fc-fusion, SARS-VHH-72-Fc. This modified VHH antibody showed a high binding affinity to SARS-CoV-2 RBD and was able to neutralize SARS-CoV-2-(S) spike glycoprotein pseudoviruses, providing further evidence of VHH antibodies as promising therapeutics in the fight against the current SARS-CoV-2 pandemic.1
Benefits of VHH Antibodies
VHH antibodies are the isolated VHH domains of heavy-chain only IgG2 and IgG3 antibodies produced by camelid species, such as alpaca, llama, and camel. These proteins consist of framework regions and three highly variable loops; H1, H2 and H3. Research has shown that it is the H3 loop that enables VHH antibodies to have such a wide range of bind specificities; the H3 loop tends to be 3–4 residues longer than that of conventional antibodies, allowing them to use these protrusions to extend into “hidden” epitope cavities; the H3 loop also has more variation in amino acid sequences in VHH antibodies than conventional antibodies, leading to ~7% higher sequence diversity per residue.6
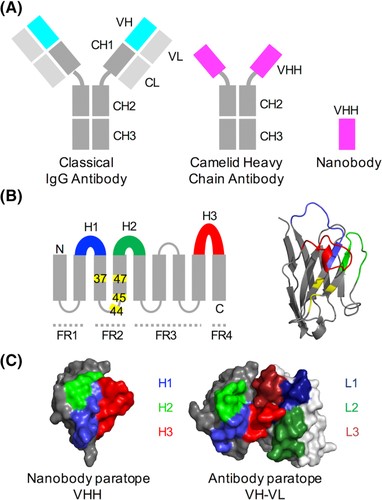
Figure 2. Structural features of conventional and camelid heavy‐chain antibodies (Ab). A, In an Ab, the antigen binds to the VH‐VL interface, while in the camelid heavy‐chain antibody the VH‐homologous VHH domain binds the antigen. In Abs, the VH and VL domains bind to each other and can only be produced in bacterial expression systems when joined by a peptide linker. B, Like the Ab VH domain, the secondary structure of the VHH domain consists of 9 beta-sheets separated by loop regions, 3 of which are hypervariable (shown in blue, green and red). Four framework regions (FRs) separate the variable loops; these are less sequence‐variable. Four positions known as the VHH‐tetrad are numbered and highlighted in yellow. Right: VHH domain with VHH‐tetrad positions in yellow. C, The antigen‐binding surface in VHH domains and Ab VH‐VL domains (aligned orientations). Image and figure legend courtesy of Mitchell & Cowell (2018)6 https://doi.org/10.1002/prot.25497 CC BY 4.0
In addition to their structural difference, there are several other features of VHH antibodies that make them more advantageous compared to canonical antibodies:3–7
| Variable functionality | VHH domain acts as a framework for recombinant antibody architecture, allowing the engineering of different structural formats. Tags can be added for purification or functional groups can be attached for the conjugation of drugs or fluorescent probes. |
|---|---|
| Small size (12–15 kDa) | Less susceptible to steric hindrance due to their smaller size, conferring improved access to epitopes. VHH antibodies have good tissue permeability and can cross the blood-brain barrier, making them attractive drug delivery options. |
| Low production cost | VHH antibodies can be produced recombinantly at high yields using bacterial, yeast, or mammalian expression systems. |
| High stability | VHH antibodies have good solubility, high thermal and chemical stability, and higher resistance to proteases than conventional antibodies. High stability also allows nebulization and administration via an inhaler to directly reach the site of infection. |
Jackson ImmunoResearch Enables the Production of High Affinity VHH Antibodies
When engineering VHH antibodies, production optimization, isolation and purification are essential to maximizing yield and generating high affinity, high-quality products that can be used to treat patients. Jackson ImmunoResearch Laboratories, Inc. specializes in producing highly specific secondary antibodies and immunoreagents and is committed to supporting manufacturers generating VHH antibodies for diagnostics or therapeutics in the treatment of novel viral infections, such as SARS-CoV-2.
Jackson ImmunoResearch has developed a range of products to facilitate the production of VHH antibodies, enabling optimization at each stage of development, from tracking seroconversion through to screening VHH antibody candidates. Their Anti-Alpaca antibodies are suitable for the detection of both alpaca, llama and camel proteins and are available with specificity for IgG (H+L), IgG, subclasses 2+3, or VHH domains. They have utility across multiple techniques such as Western blotting, ELISA, flow cytometry, and immunofluorescence. These products have been designed to optimize VHH antibody discovery to ensure the production of high-quality candidates.7,8
References and Further Reading
- Wrapp D., et al. (2020). Structural Bases for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. https://doi.org/10.1016/j.cell.2020.04.031.
- www.bbc.com. (2020). Coronavirus Pandemic: Tracking the Global Outbreak. https://www.bbc.co.uk/news/world-51235105?utm_source=dlvr.it&utm_medium=twitter
- Mahase E. (2020). Covid-19: What Treatments are Being Investigated? British Medical Journal. doi: 10.1136/bmj.m1252.
- Zhao G., et al. (2018). A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy Against MERS-COV. Journal of Virology. https://doi.org/10.1128/JVI.00837-18.
- https://www.nih.gov/news-events/nih-research-matters/potent-antibodies-found-people-recovered-covid-19
- Mitchell L. S., et al. (2017). Comparative Analysis of Nanobody Sequence and Structure Data. Proteins. https://doi.org/10.1002/prot.25497.
- www.jacksonimmuno.com. (2020). Camelid Immunology. https://www.jacksonimmuno.com/technical/products/groups/camelid
- www.jacksonimmuno.com. (2020). Introducing Anti-Alpaca IgG, VHH Domain Secondary Antibodies. https://www.jacksonimmuno.com/secondary-antibody-resource/wp-content/uploads/Anti-VHH-handout-US-online.pdf
- Zhang Z., et al (2020). Human neutralizing antibodies elicited by SARS-CoV-2 infection. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature PubMed: 32454513 DOI: 10.1038/s41586-020-2380-z


