
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
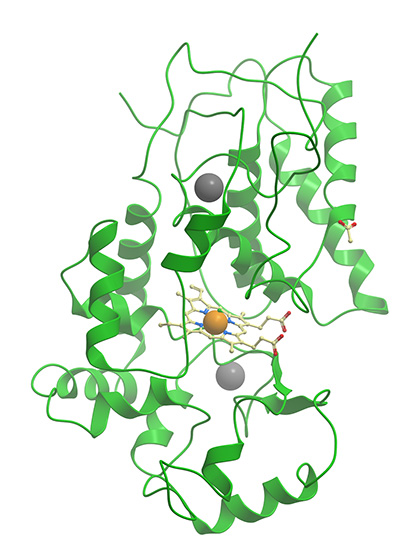
This commonly used reporter enzyme is derived from the root of the horseradish plant (Armoracia rusticana). JIR HRP conjugates are prepared by a modified Nakane and Kawaoi procedure (1974).
HRP conjugates are suitable for all immunotechniques employing colorimetric and chemiluminescent detection methods, including Western blotting, immunohistochemistry and ELISA. Jackson ImmunoResearch provides a wide range of secondary antibodies, with comprehensive options for host and target species, as well as immunoglobulin specificities.
Horseradish peroxidase is available conjugated to:
We also offer Peroxidase-Anti-Peroxidase (PAP) as as an alternative labeling approach.
Some tissues contain endogenous peroxidase-like enzymes which can react with peroxidase substrates, resulting in background staining. Pre-treatment of sample with hydrogen peroxidase will exhaust the endogenous enzyme activity, allowing clear detection of specific signal.
HRP conjugates can be used for colorimetric detection. The conjugated reporter enzyme catalyzes the conversion of the chromogenic substrate to a colored precipitate, visualized directly on the blotting membrane or tissue sample. Colorimetric detection can offer quick and easily obtained results without the need for expensive detectors or extensive optimization.
Enzyme-linked conjugates can also be used for chemiluminescent signal detection. HRP conjugates produce signal by oxidizing a chemiluminescent substrate (luminol) to a form which emits light. AP conjugates produce signal when the enzyme dephosphorylates a specific substrate (e.g. 1,2-dioxetane) to a light emitting product. The signal can then be captured by exposing photographic film to the membrane or using a cooled charge-coupled device (CCD) camera. Chemiluminescent detection offers excellent sensitivity, however quantification and probing for multiple targets can be limited, and development may require refinement to optimize signal capture.
Affinity-purified anti-horseradish peroxidase antibodies are available for detection of horseradish peroxidase antigen, or for signal amplification of HRP-containing reagents. For immunostaining of mammalian cells, an advantage of using anti-horseradish peroxidase is reduced background, since the antibody does not recognize the endogenous peroxidase-like enzymes found in those cells.